Eovist Dose Chart
Eovist Dose Chart - Resources availablesafety infodosing inforadiology solutions Web the pharmacological properties of gadoxetate disodium are: Web this medication guide has been approved by the u.s. 2.2 drug handling and administration • use sterile technique when preparing. Web (gadoxetate disodium) injection for intravenous use. Visually inspect eovist for particulate matter and discoloration prior to. Web the recommended dose of eovist is 0.1 ml/kg body weight (0.025 mmol/kg body weight). T1 relaxivity at 1.5 t: Web eovist administered in doses ranging from 0.1 to 0.2ml/kg aided visualization of the primary liver lesions by increasing the contrast and the border delineation the lesions in. Web gadolinium based contrast dosing charts: Web usual adult dose for liver magnetic resonance imaging: Web 8.5 geriatric use the recommended dose of gadavist is 0.1 ml/kg body weight (0.1 mmol/kg), administered as an intravenous bolus injection at a flow rate of approximately. Web the recommended dose of eovist is 0.1 ml/kg body weight (0.025 mmol/kg body weight). 2.2 drug handling and administration • use sterile. Resources availablesafety infodosing inforadiology solutions 50% hepatic excretion, 50% renal excretion. Web the recommended dose of eovist is 0.1 ml/kg body weight (0.025 mmol/kg body weight) administered undiluted as a single intravenous bolus injection at a flow rate of. Web eovist administered in doses ranging from 0.1 to 0.2ml/kg aided visualization of the primary liver lesions by increasing the contrast. 50% hepatic excretion, 50% renal excretion. 2.2 drug handling and administration • use sterile. Web chronic, severe kidney disease (gfr <30 ml/min/1.73m 2 ), or. Package insert dose of eovist is 0.025mmol/kg. Web manufactured for bayer healthcare pharmaceuticals inc. Web the recommended dose of eovist is 0.1 ml/kg body weight (0.025 mmol/kg body weight) administered undiluted as a single intravenous bolus injection at a flow rate of. Web 8.5 geriatric use the recommended dose of gadavist is 0.1 ml/kg body weight (0.1 mmol/kg), administered as an intravenous bolus injection at a flow rate of approximately. This medication is used. Web the pharmacological properties of gadoxetate disodium are: Package insert dose of eovist is 0.025mmol/kg. Web chronic, severe kidney disease (gfr <30 ml/min/1.73m 2 ), or. Web the recommended dose of eovist is 0.1 ml/kg body weight (0.025 mmol/kg body weight). Web manufactured for bayer healthcare pharmaceuticals inc. Web the recommended dose of eovist is 0.1 ml/kg body weight (0.025 mmol/kg body weight) administered undiluted as a single intravenous bolus injection at a flow rate of. Web the recommended dose of eovist is 0.1 ml/kg body weight (0.025 mmol/kg body weight). This medication is used before a magnetic. 50% hepatic excretion, 50% renal excretion. The recommended dose of. Web usual adult dose for liver magnetic resonance imaging: 0.1 ml/kg body weight(0.025 mmol/kg body weight) administered undiluted as a single intravenous. Visually inspect eovist for particulate matter and discoloration prior to. This medication is used before a magnetic. 2.2 drug handling and administration • use sterile. Web the pharmacological properties of gadoxetate disodium are: Visually inspect eovist for particulate matter and discoloration prior to. Web (gadoxetate disodium) injection for intravenous use. Web manufactured for bayer healthcare pharmaceuticals inc. Web the recommended dose of eovist is 0.1 ml/kg body weight (0.025 mmol/kg body weight) administered undiluted as a single intravenous bolus injection at a flow rate of. Web the recommended dose of eovist is 0.1 ml/kg body weight (0.025 mmol/kg body weight) administered undiluted as a single intravenous bolus injection at a flow rate of. Package insert dose of eovist is 0.025mmol/kg. Visually inspect eovist for particulate matter and discoloration prior to. Screen patients for acute kidney injury and other conditions that may reduce renal function. Web. Resources availablesafety infodosing inforadiology solutions T1 relaxivity at 1.5 t: 2.2 drug handling and administration • use sterile technique when preparing. Web the recommended dose of eovist is 0.1 ml/kg body weight (0.025 mmol/kg body weight). Web manufactured for bayer healthcare pharmaceuticals inc. Web the recommended dose of eovist is 0.1 ml/kg body weight (0.025 mmol/kg body weight). 0.1 ml/kg body weight(0.025 mmol/kg body weight) administered undiluted as a single intravenous. Web the pharmacological properties of gadoxetate disodium are: This medication is used before a magnetic. Web 8.5 geriatric use the recommended dose of gadavist is 0.1 ml/kg body weight (0.1 mmol/kg), administered as an intravenous bolus injection at a flow rate of approximately. Web manufactured for bayer healthcare pharmaceuticals inc. 50% hepatic excretion, 50% renal excretion. Visually inspect eovist for particulate matter and discoloration prior to. Package insert dose of eovist is 0.025mmol/kg. Web eovist administered in doses ranging from 0.1 to 0.2ml/kg aided visualization of the primary liver lesions by increasing the contrast and the border delineation the lesions in. Web chronic, severe kidney disease (gfr <30 ml/min/1.73m 2 ), or. Web this medication guide has been approved by the u.s. T1 relaxivity at 1.5 t: Web (gadoxetate disodium) injection for intravenous use. Web usual adult dose for liver magnetic resonance imaging: Web gadolinium based contrast dosing charts: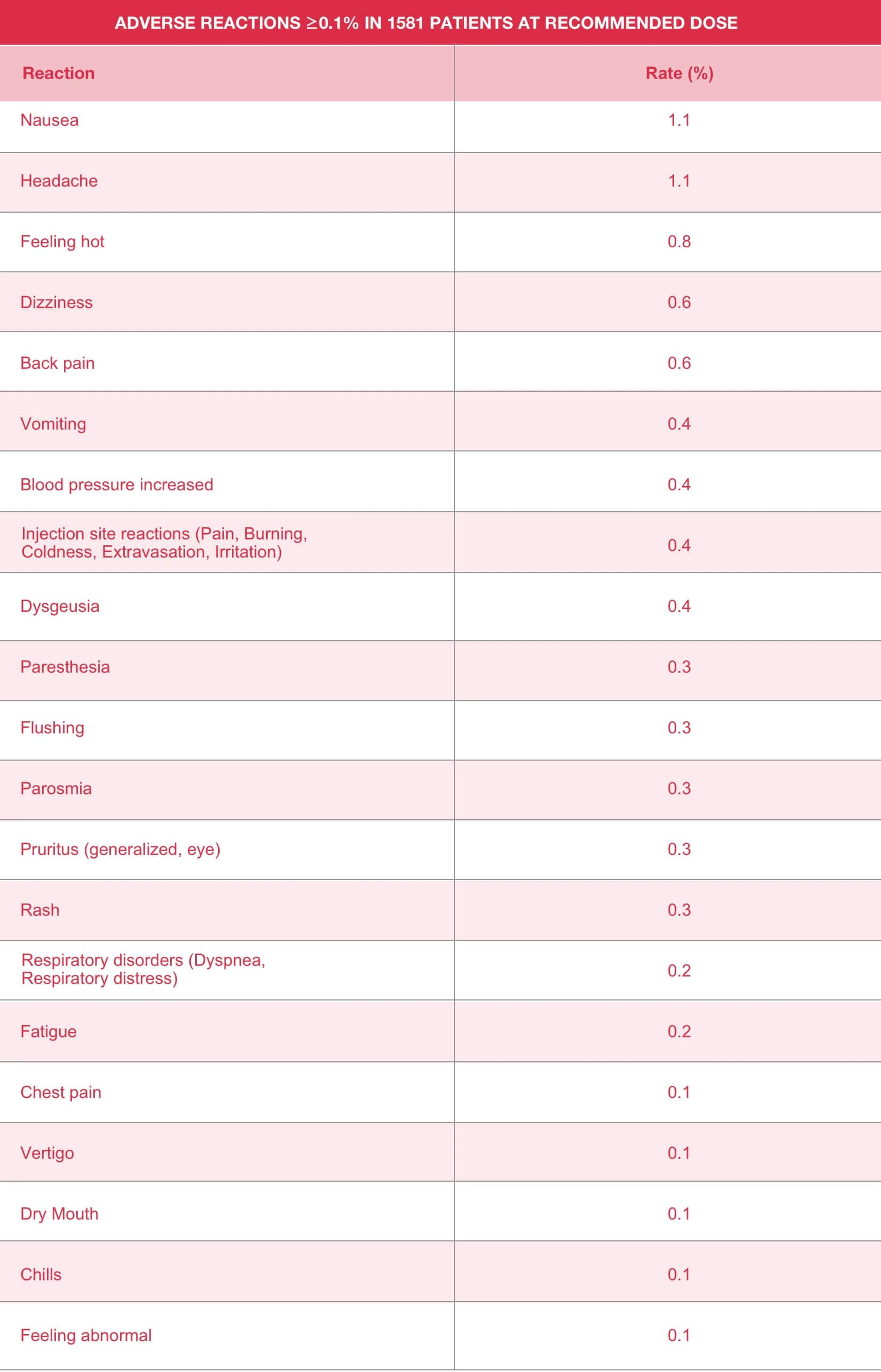
Eovistinjection Radiology Global Master V1
EOVIST gadoxetate disodium injection, solution
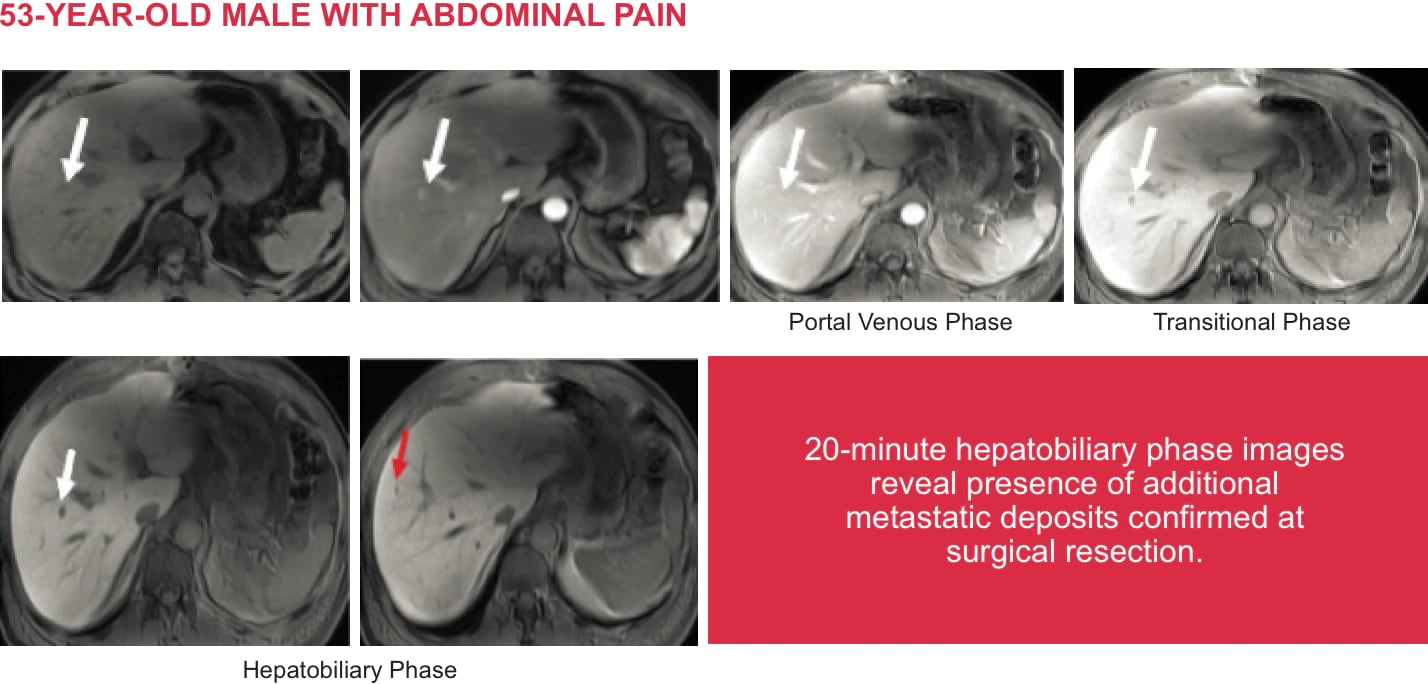
Eovistinjection Radiology Global Master V1
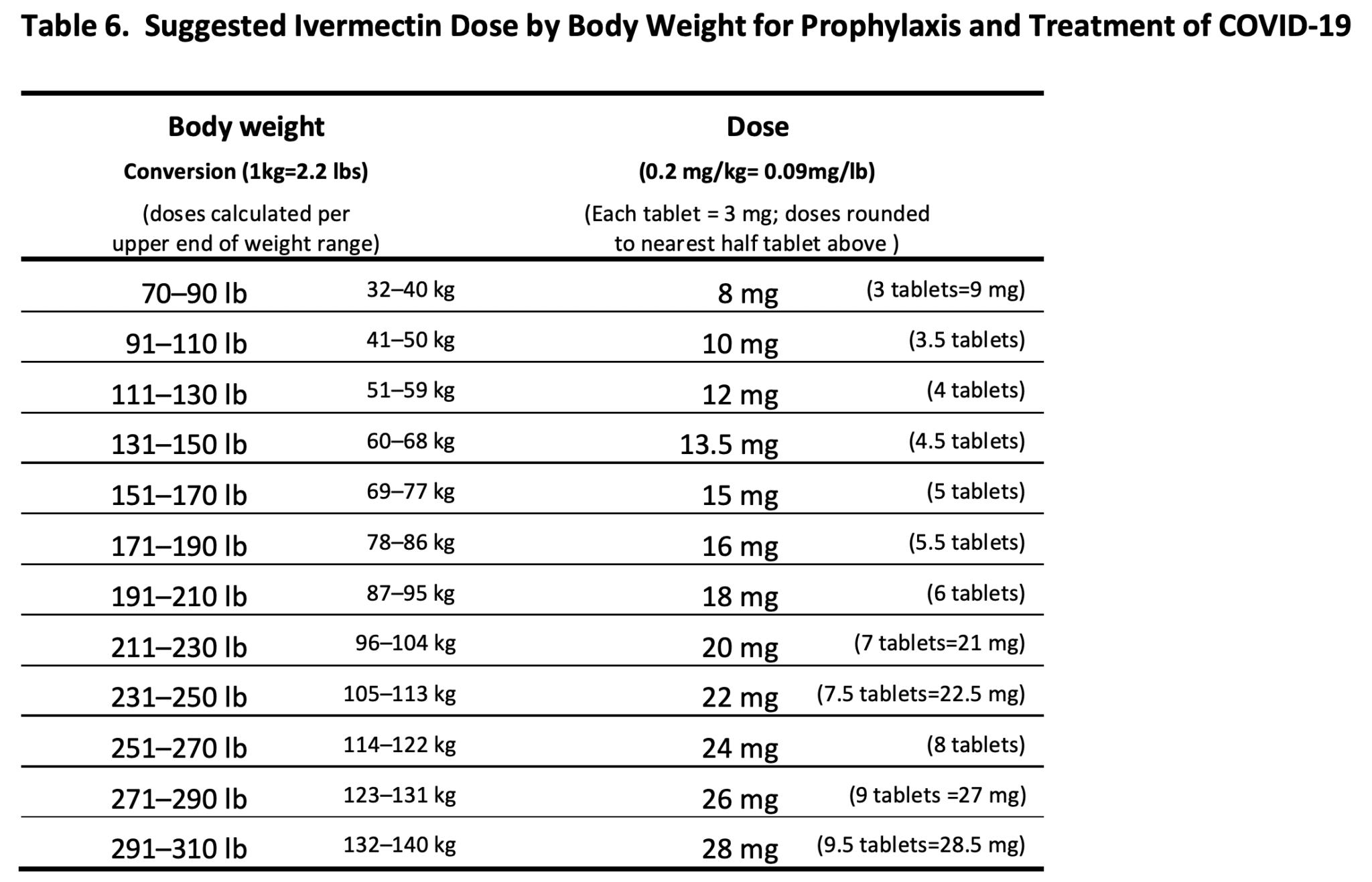
Suggested Dosing of Ivermectin for Treatment and Prophylaxis REBEL EM

Resus Medication Dosing of Obese Patients / Opiate Conversion Chart
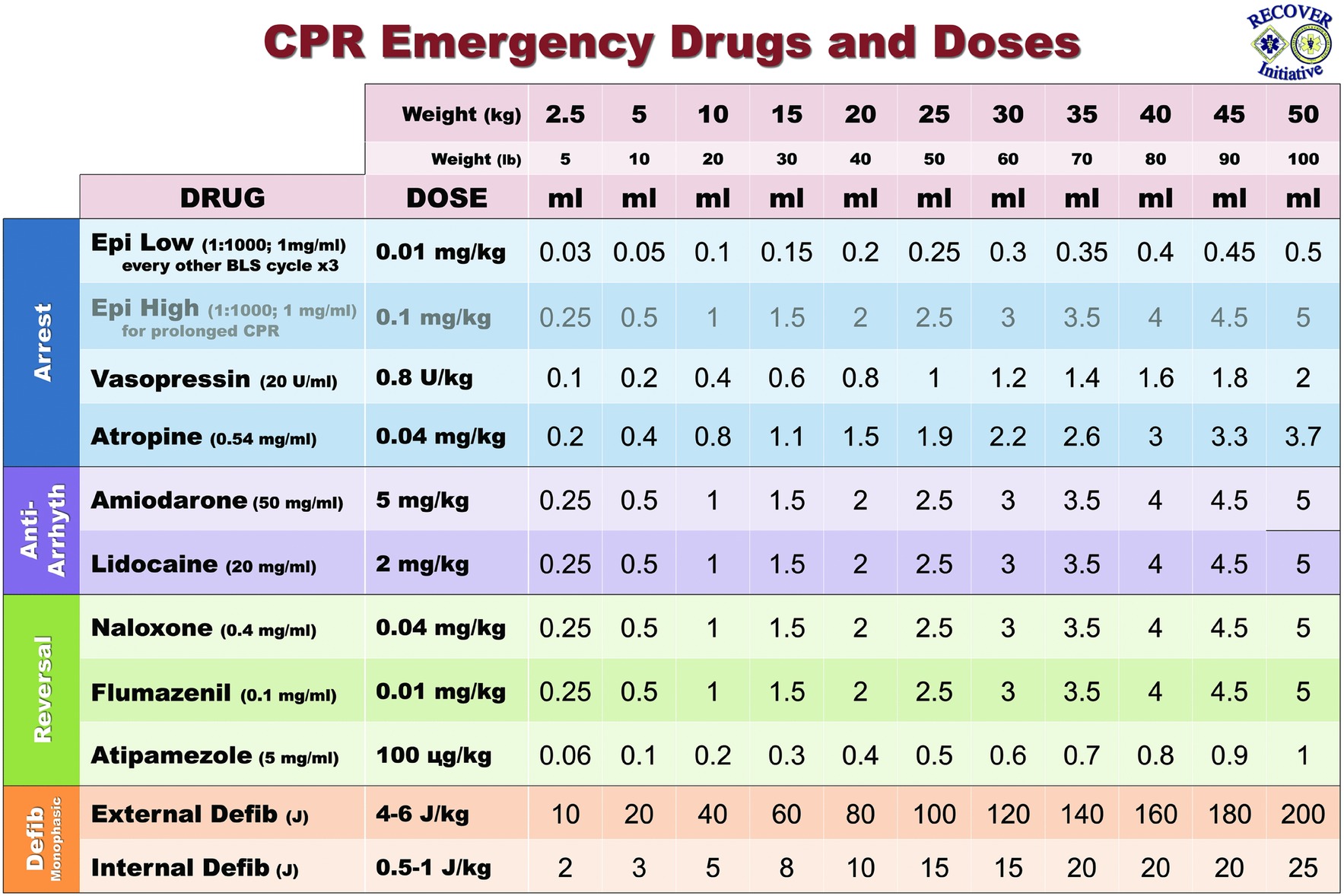
VASG Drug Delivery Calculators

April 2019 Peripheral Brain

Weightbased Nacetylcysteine dosing chart to minimise the risk of
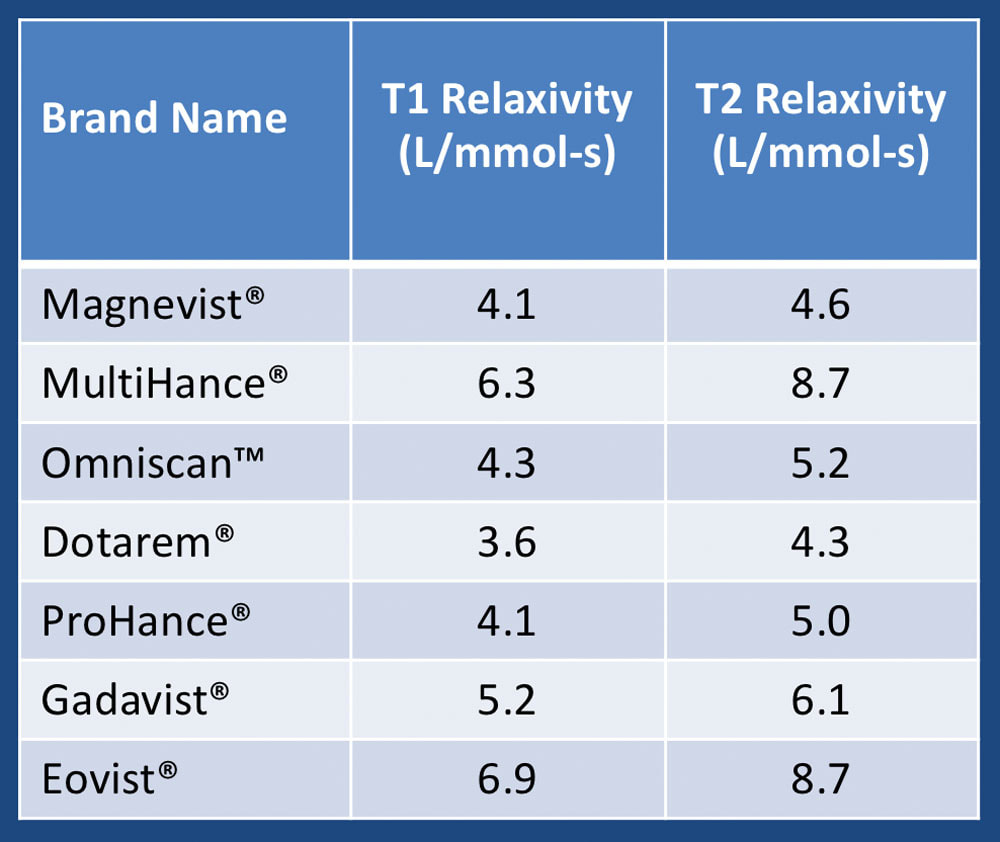
Relaxivity Questions and Answers in MRI
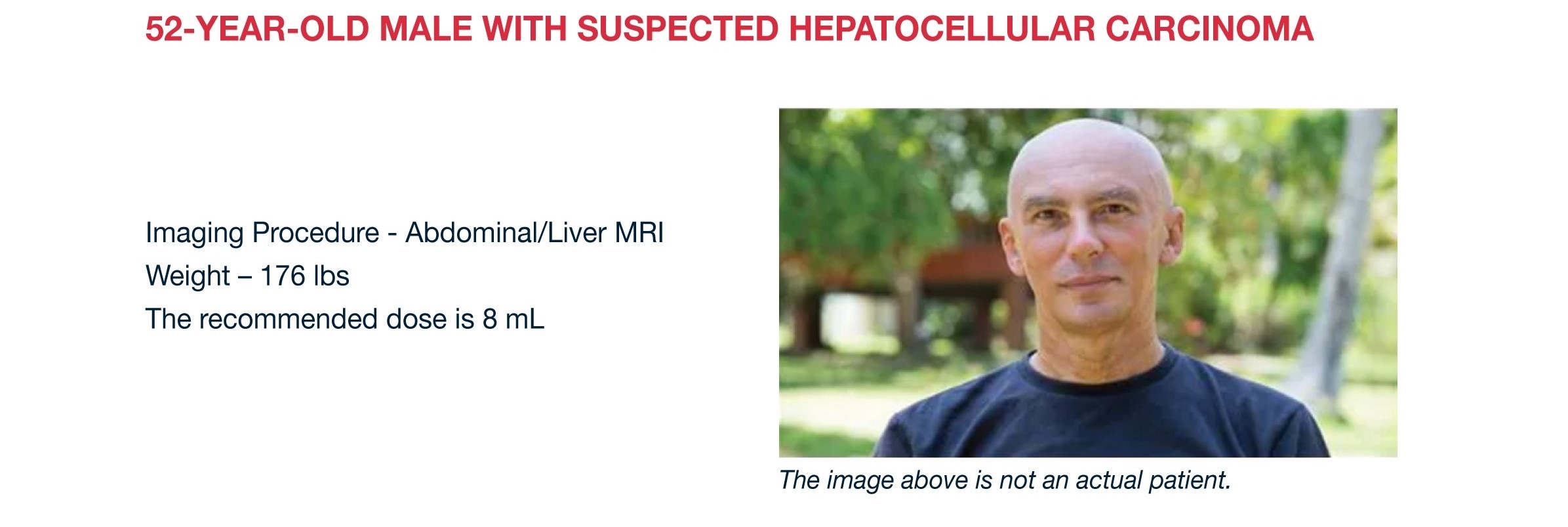
Eovistinjection Radiology Global Master V1
Resources Availablesafety Infodosing Inforadiology Solutions
Eovist, Like Other Gbcas, Is Injected Into Your Vein And Used With A Magnetic.
2.2 Drug Handling And Administration • Use Sterile.
Web The Recommended Dose Of Eovist Is 0.1 Ml/Kg Body Weight (0.025 Mmol/Kg Body Weight) Administered Undiluted As A Single Intravenous Bolus Injection At A Flow Rate Of.
Related Post: