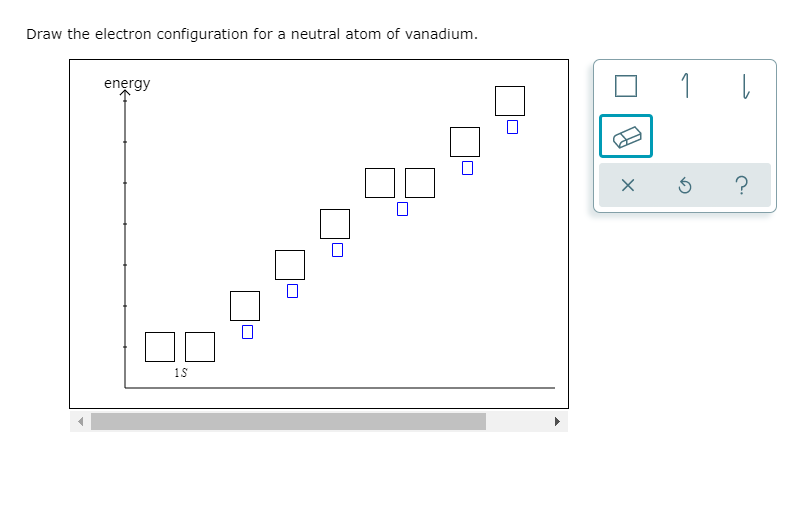
Draw The Electron Configuration For A Neutral Atom Of Fluorine
Draw The Electron Configuration For A Neutral Atom Of Fluorine - Web find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it,. If fluorine becomes an ion with a charge of 1−, what will its orbital diagram look like in the ground state? 1 ), and the electron configuration is written as 1 s1. As the atomic number is equal to the number of electrons present in a neutral. Write the full electron configuration for a neutral fluorine atom. Web write the full electron configuration for a neutral fluorine atom. Web a neutral fluorine atom has 9 protons and 9 electrons. Web study with quizlet and memorize flashcards containing terms like what is the electron configuration of the neutral lithium atom?, what is the electron configuration. Web first, write out the electron configuration for each parent atom. Web study with quizlet and memorize flashcards containing terms like what is the electron configuration of the neutral lithium atom?, what is the electron configuration. We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it,. So fluorine has a total of nine electrons so starting from the beginning it's. 1s22s22p5 draw the. Web for hydrogen, therefore, the single electron is placed in the 1 s orbital, which is the orbital lowest in energy (figure 6.8.1 6.8. As the atomic number is equal to the number of electrons present in a neutral. 1s 2 2s 2 2p 5. Web a neutral fluorine atom has 9 protons and 9 electrons. Web its electron configuration. Web write the full electron configuration for a neutral fluorine atom. Web solution for write the full electron configuration for a neutral fluorine atom. Okay, let's write out and then draw the electron configuration for fluorine. In this article, we will study how electrons are arranged in different shells and subshells in the fluorine atom. So fluorine has a total. Web the symbol of fluorine is f. Web study with quizlet and memorize flashcards containing terms like what is the electron configuration of the neutral lithium atom?, what is the electron configuration. An orbital diagram is used to determine an atom’s electron. Within each shell, the s subshell is at a lower energy than the p. Write the full electron. Web the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. 1s 2s22p draw the lewis dot symbol for a neutral fluroine atom identify. Web the electron configuration of a fluorine nucleus, f,f, with 9 electrons is 1s22s22p5. Atomic number of fluorine is 9 and it is. Web the electron configuration of fluorine is: Atomic number of fluorine is 9 and it is a part of halogen family. Web #1s^(2)2s^(2)2p^5#.the fact that fluorine almost has a full valence shell, i.e. Web the symbol of fluorine is f. Web find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. The elements of group 17 have seven electrons in their outermost shell. 1 ), and the electron configuration is written as 1 s1. So fluorine has a total of nine electrons so starting from the beginning it's. An orbital diagram is used to determine an atom’s electron. Electronic configuration of group 17: Web find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. An orbital diagram is used to determine an atom’s electron. Web a neutral fluorine atom has 9 protons and 9 electrons. Atomic number of fluorine is 9 and it is a part of halogen family. Web #1s^(2)2s^(2)2p^5#.the fact that fluorine almost has. Web the electron configuration of fluorine is: Web solution for write the full electron configuration for a neutral fluorine atom. Okay, let's write out and then draw the electron configuration for fluorine. Web first, write out the electron configuration for each parent atom. Web #1s^(2)2s^(2)2p^5#.the fact that fluorine almost has a full valence shell, i.e. Web a neutral fluorine atom has 9 protons and 9 electrons. Web its electron configuration is 1s 2 2s 2 2p 5. Web write the full electron configuration for a neutral fluorine atom. Web the symbol of fluorine is f. 1s 2 2s 2 2p 5. Write the full electron configuration for a neutral fluorine atom. Web a neutral fluorine atom has 9 protons and 9 electrons. Electronic configuration of group 17: Web write the full electron configuration for a neutral fluorine atom. Web fluorine (atomic number 9) has only one 2porbital containing an unpaired electron. Web study with quizlet and memorize flashcards containing terms like what is the electron configuration of the neutral lithium atom?, what is the electron configuration. Write the full electron configuration for a neutral fluorine atom. This makes it easier to. Draw the lewis dot symbol for a neutral fluroine… A fluorine atom has 9 protons, so a fluorine nucleus with 9 electrons is a neutral fluorine. Web solution for write the full electron configuration for a neutral fluorine atom. Web the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. 1s 2 2s 2 2p 5. Web first, write out the electron configuration for each parent atom. If fluorine becomes an ion with a charge of 1−, what will its orbital diagram look like in the ground state? As the atomic number is equal to the number of electrons present in a neutral.
3.4 Electronic Structure of Atoms (Electron Configurations) General
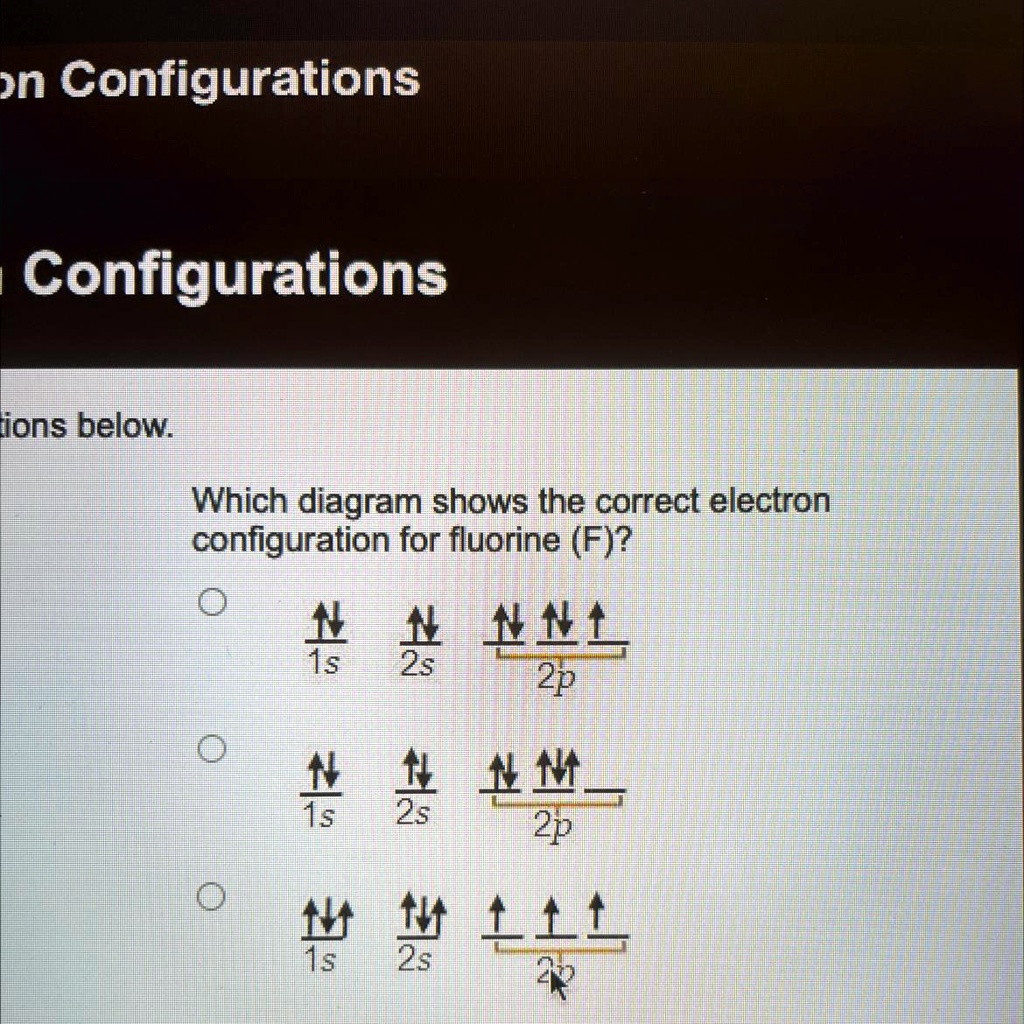
SOLVED Which diagram shows the correct electron configuration for

The Electron Configuration of Fluorine YouTube
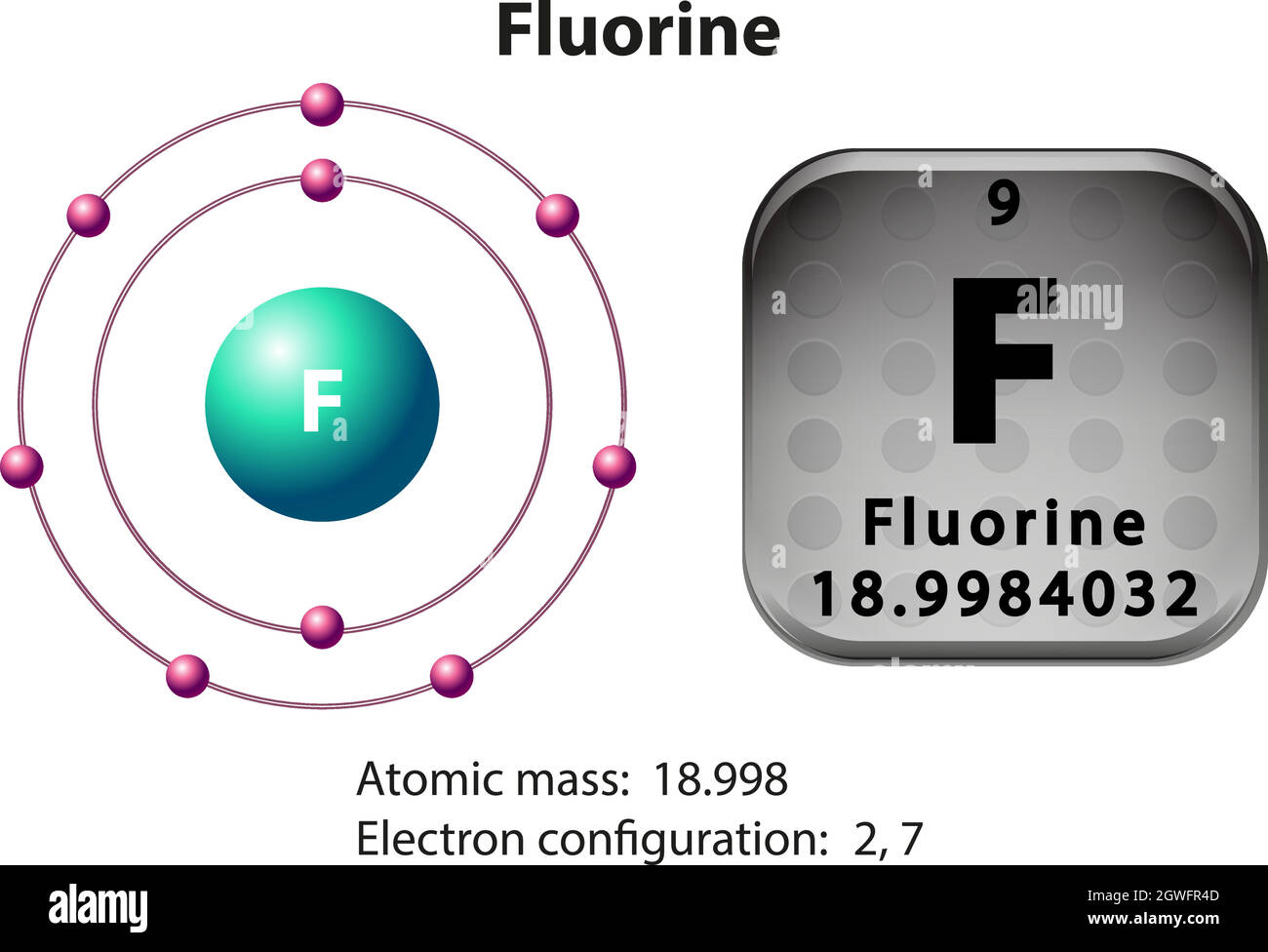
Symbol and electron diagram for Fluorine Stock Vector Image & Art Alamy

Fluorine Electron Configuration Diagram vrogue.co
Solved Draw the electron configuration for a neutral atom of
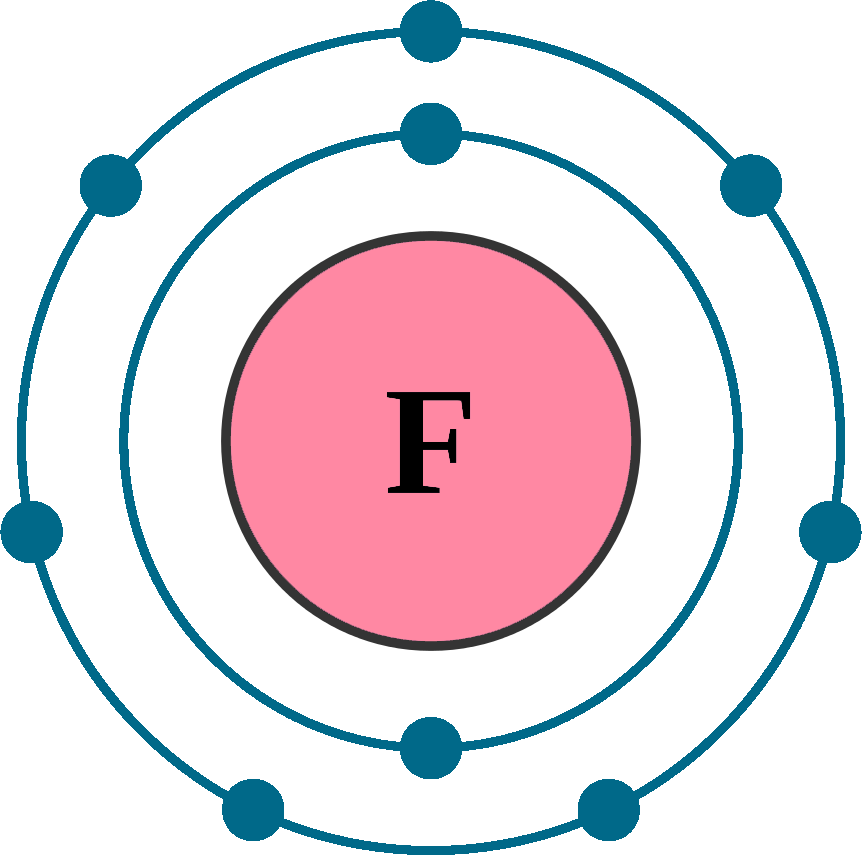
Fluorine Electron Configuration Diagram

How many valence electrons does fluorine(F) have?

Draw The Electron Configuration For A Neutral Atom Of Fluorine
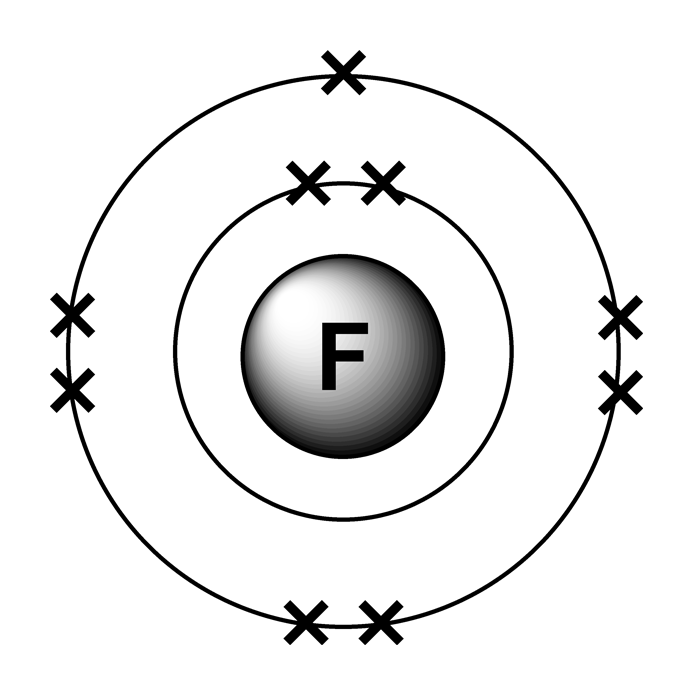
Electron arrangements
Web The Symbol Of Fluorine Is F.
So Fluorine Has A Total Of Nine Electrons So Starting From The Beginning It's.
Web The Electron Configuration Of Fluorine Is:
1S 2S22P Draw The Lewis Dot Symbol For A Neutral Fluroine Atom Identify.
Related Post: