What Is Design Control
What Is Design Control - Understand controls and evaluate design includes the following planning forms, each a component of internal control as identified by coso: Web § 820.30 design controls. • design and development planning
• design input, including intended use and user needs (also known as customer attributes) They are a systematic way to assure that medical. Web design control is a rigorous process covering the definition of design requirements denoted as design inputs, design, design review, verification that the final device meets. Learn the elements, documents, and best practices. Web since assessing the design of the control is the first step, auditors need to understand the control objective and associated control activities and ensure that all. Web design control is the area of requirements from the us quality system regulation or qsr, that apply to the design and development of medical devices. Web to move to the internal control section, beginning with understand controls and evaluate design. Design controls for medical devices are, in short, a series of structured requirements that facilitate a compliant design and. Web design control is a rigorous process covering the definition of design requirements denoted as design inputs, design, design review, verification that the final device meets. Web all the ways ios 18 lets you customize your home screen, control center, and more. They are a systematic way to assure that medical. Since 1990, the food and drug administration (fda) has. They are a systematic way to assure that medical. Web design controls and risk management should flow and blend together, and it’s important to establish this flow early in product development. Web the unique design of the cauldron pays tribute to the first flight of the hot air balloon in the tuileries garden. Understand controls and evaluate design includes each. Web learn what design controls are, why they are important, and how they apply to medical devices. Understand controls and evaluate design includes the following planning forms, each a component of internal control as identified by coso: Web since assessing the design of the control is the first step, auditors need to understand the control objective and associated control activities. At a high level, this regulation requires: Web to move to the internal control section, beginning with understand controls and evaluate design. ( 1) each manufacturer of any class iii or class ii device, and the class i devices listed in paragraph (a) (2) of this section, shall establish. Web all the ways ios 18 lets you customize your home. Web design control is the area of requirements from the us quality system regulation or qsr, that apply to the design and development of medical devices. ( 1) each manufacturer of any class iii or class ii device, and the class i devices listed in paragraph (a) (2) of this section, shall establish. • design and development planning
• design. Web the unique design of the cauldron pays tribute to the first flight of the hot air balloon in the tuileries garden. ( 1) each manufacturer of any class iii or class ii device, and the class i devices listed in paragraph (a) (2) of this section, shall establish. This guidance complements the regulation by describing its intent from a. This guide covers the global r… Web what are design controls? ( 1) each manufacturer of any class iii or class ii device, and the class i devices listed in paragraph (a) (2) of this section, shall establish. Web learn what design controls are, why they are important, and how they apply to medical devices. Web to move to the. The assurance process includes a total systems approach from the. • design and development planning
• design input, including intended use and user needs (also known as customer attributes) Web all the ways ios 18 lets you customize your home screen, control center, and more. Web what are design controls? To be included, a trial had to focus on critically. To be included, a trial had to focus on critically ill adults (≥ 18 years) and compare outcomes between those randomized to intravenous insulin. Web what are design controls? At a high level, this regulation requires: ( 1) each manufacturer of any class iii or class ii device, and the class i devices listed in paragraph (a) (2) of this. Web the unique design of the cauldron pays tribute to the first flight of the hot air balloon in the tuileries garden. Web design control is a systematic approach to the development and production of products, particularly medical devices. Understand controls and evaluate design includes each component of. The biggest headline of ios 18 is undoubtedly apple intelligence, but if.. Understand controls and evaluate design includes the following planning forms, each a component of internal control as identified by coso: Learn the elements, documents, and best practices. Web design controls and risk management should flow and blend together, and it’s important to establish this flow early in product development. Web what are design controls? Web all the ways ios 18 lets you customize your home screen, control center, and more. Web the unique design of the cauldron pays tribute to the first flight of the hot air balloon in the tuileries garden. Web design control is a systematic approach to the development and production of products, particularly medical devices. ( 1) each manufacturer of any class iii or class ii device, and the class i devices listed in paragraph (a) (2) of this section, shall establish. Web design control is the area of requirements from the us quality system regulation or qsr, that apply to the design and development of medical devices. The assurance process includes a total systems approach from the. This guide covers the global r… Web design controls are a set of quality practices and procedures incorporated into the design and development process. Web to move to the internal control section, beginning with understand controls and evaluate design. Web design controls are based upon quality assurance and engineering principles. Learn what design controls are, how they apply to different types of medical devices, and how to implement them effectively. They are a systematic way to assure that medical.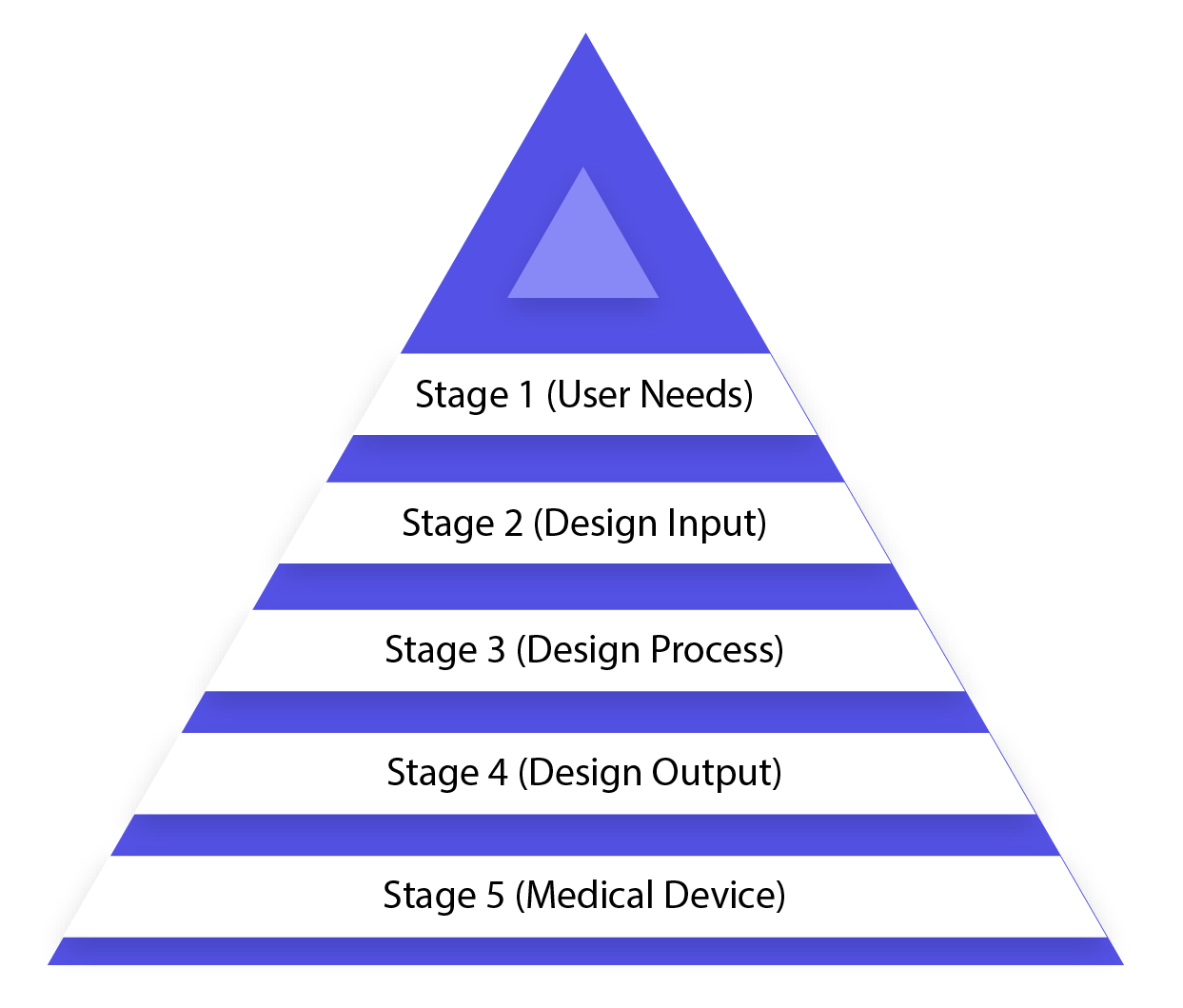
FDA Design Control The Ultimate Guide For Medical Device Companies
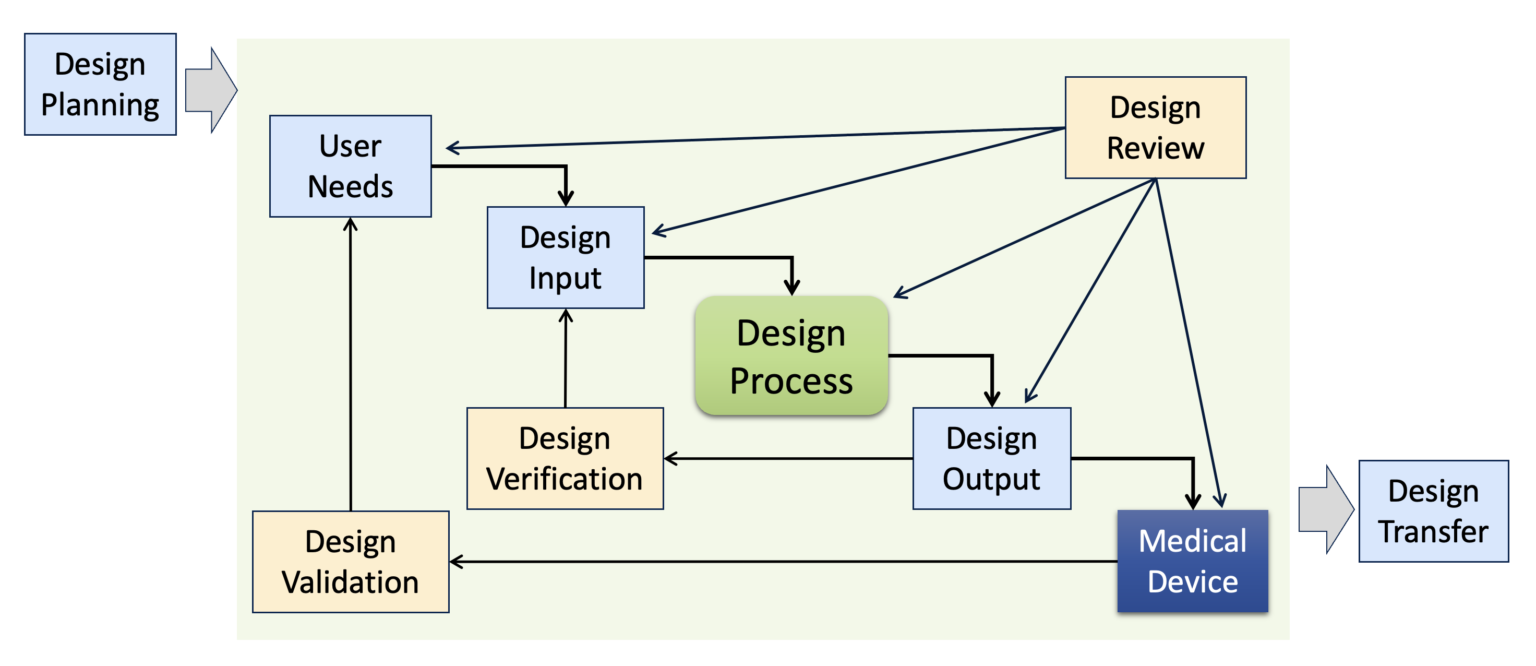
What Are Medical Device Design Controls? Sunstone Pilot, Inc.
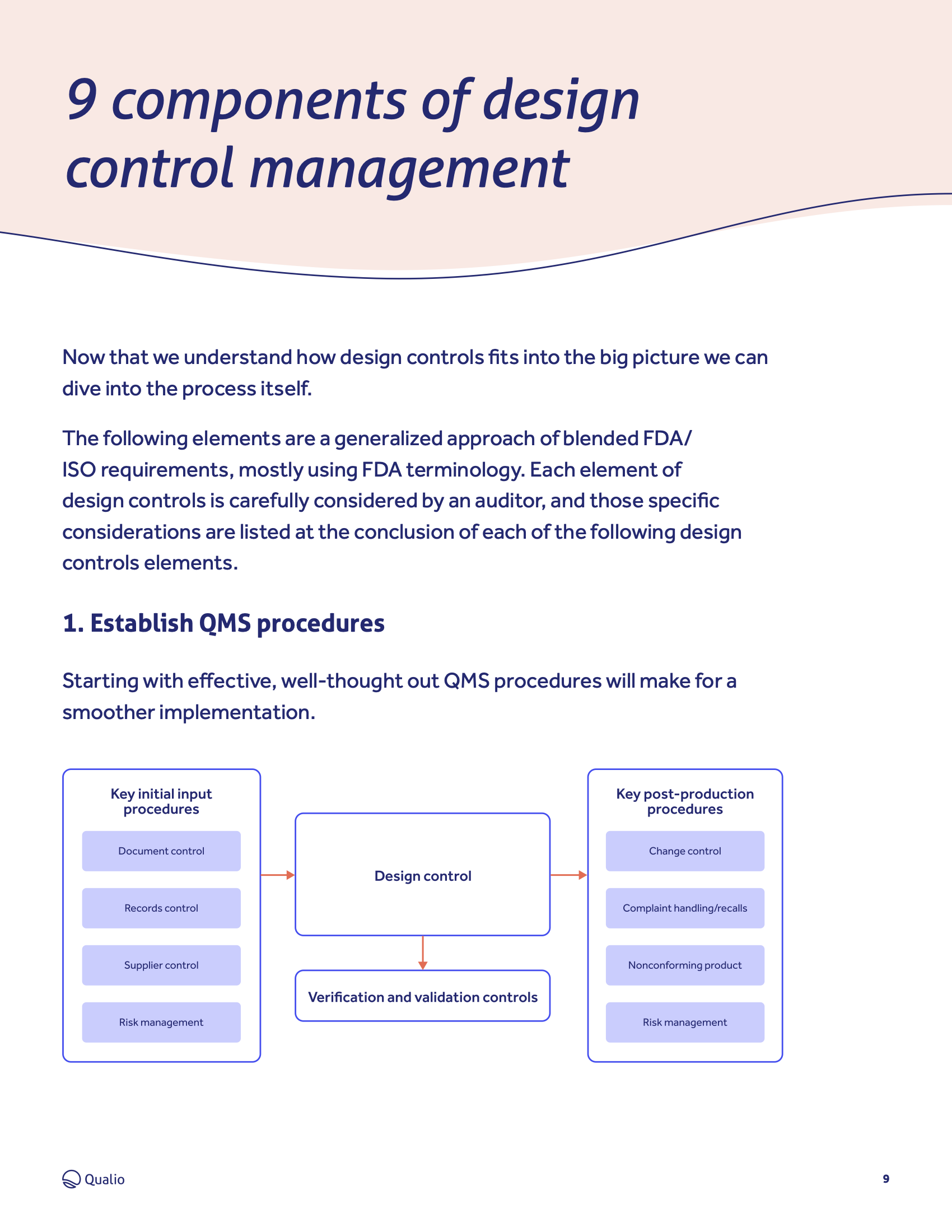
Ultimate guide to medical device design controls

Basics of Medical Device Design Controls What, Why, and How Oriel
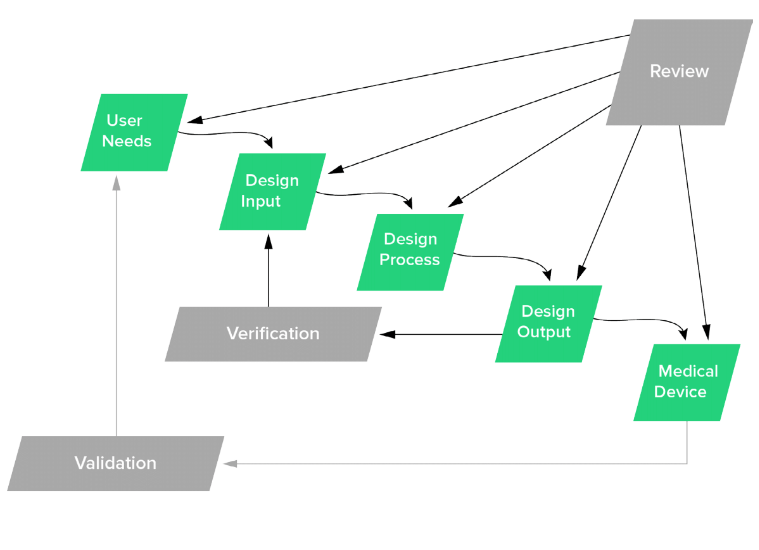
The Ultimate Guide To Design Controls For Medical Device Companies

Design Controls Digest 5 Phases of Design Controls You Need to Know

Design Controls Digest 5 Phases of Design Controls You Need to Know

5 Key Design Control Best Practices Jama Software
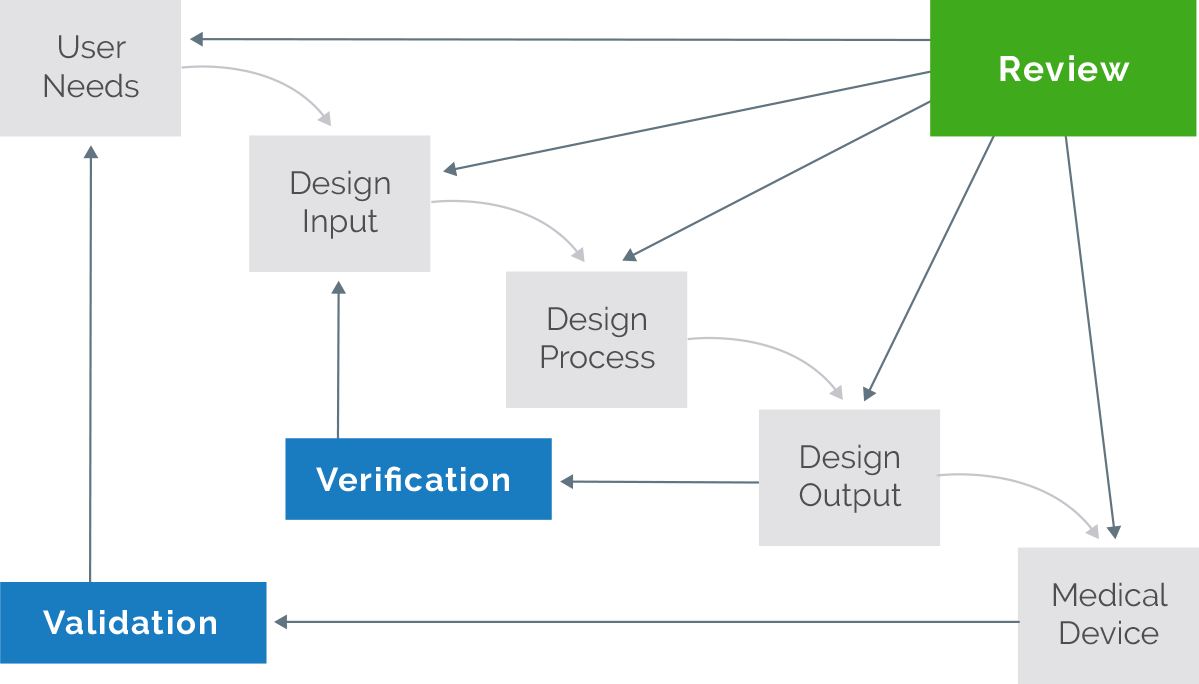
Design Controls Definition Arena
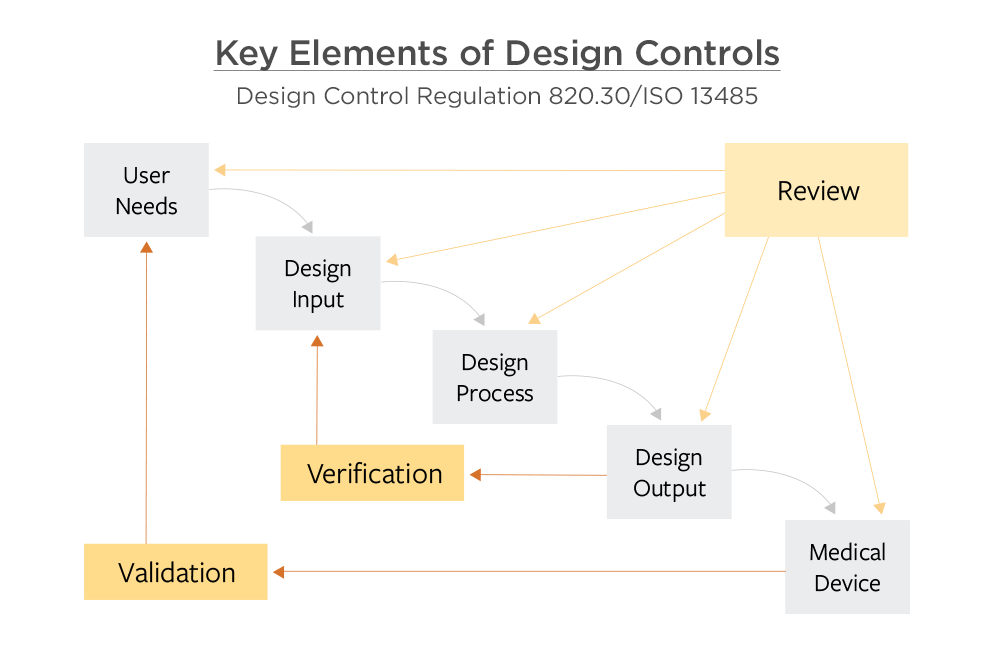
Good Design Controls Are Critical to Avoid FDA Issues Arena
To Be Included, A Trial Had To Focus On Critically Ill Adults (≥ 18 Years) And Compare Outcomes Between Those Randomized To Intravenous Insulin.
• Design And Development Planning
• Design Input, Including Intended Use And User Needs (Also Known As Customer Attributes)
Web § 820.30 Design Controls.
Since 1990, The Food And Drug Administration (Fda) Has Required That Medical Device Manufacturers That Want To Market Certain Categories Of Medical Devices In The Usa Follow Design Control Requirements (21 Cfr 820.30).
Related Post: