The Stereochemical Designators And Distinguish Between
The Stereochemical Designators And Distinguish Between - Enantiomers at an epimeric carbon atom b. Incorporate the e,z designation into the iupac name of a given alkene. Web the stereochemical designators α and β distinguish between: Web chemists need a convenient way to distinguish one stereoisomer from another. Web differences in the spatial orientation of their constituent atoms. Enantiomers at an epimeric carbon atom. Web the study of stereochemistry focuses on the relationships between stereoisomers, which by definition have the same molecular formula and sequence of bonded atoms. The stereochemical designators alpha and beta distinguish between: The stereochemical designators α and β distinguish between: Web diastereomers are stereoisomers with two or more chiral centers that are not enantiomers. Web study with quizlet and memorize flashcards containing terms like the stereochemical designators α and β distinguish between which stereoisomers?, how does na+ flow. Web diastereomers are stereoisomers with two or more chiral centers that are not enantiomers. Enantiomers at an epimeric carbon atom. Epimers are stereoisomers that differ in their configuration at. Incorporate the e,z designation into the iupac. Web specifically, the α designator is used for substituents that are located on the same side of the molecule's carbon atom, while the β designator is used for. Web study with quizlet and memorize flashcards containing terms like the stereochemical designators α and β distinguish between which stereoisomers?, how does na+ flow. Web differences in the spatial orientation of their. Which ideal solution exhibits the greatest osmotic pressure? Web the study of stereochemistry focuses on the relationships between stereoisomers, which by definition have the same molecular formula and sequence of bonded atoms. Web the stereochemical designators alpha and beta indicate the orientation of substituents around a cyclic structure or at ring fusion sites in cyclic molecules like. Isn't this supposed. Web the stereochemical designators α and β distinguish between epimers at an anomeric carbon atom. Which ideal solution exhibits the greatest osmotic pressure? Isn't this supposed to be anomers? Epimers are stereoisomers that differ in their configuration at. Web diastereomers are stereoisomers with two or more chiral centers that are not enantiomers. Enantiomers at an anomeric carbon atom. Enantiomers at an epimeric carbon atom b. Web diastereomers are stereoisomers with two or more chiral centers that are not enantiomers. Enantiomers at an anomeric carbon atom c. Incorporate the e,z designation into the iupac name of a given alkene. Enantiomers at an anomeric carbon atom. Several various classes and properties of isomers relevant to the mcat will be examined here. Web differences in the spatial orientation of their constituent atoms. Web the stereochemical designators alpha and beta indicate the orientation of substituents around a cyclic structure or at ring fusion sites in cyclic molecules like. The stereochemical designators alpha. Epimers at the anomeric carbon. Web the study of stereochemistry focuses on the relationships between stereoisomers, which by definition have the same molecular formula and sequence of bonded atoms. Web differences in the spatial orientation of their constituent atoms. Web specifically, the α designator is used for substituents that are located on the same side of the molecule's carbon atom,. The stereochemical designators alpha and beta distinguish between: Web the stereochemical designators α and β distinguish between epimers at an anomeric carbon atom. Isn't this supposed to be anomers? The stereochemical designators α and β distinguish between: Web study with quizlet and memorize flashcards containing terms like the stereochemical designators α and β distinguish between which stereoisomers?, how does na+. Web the stereochemical designators alpha and beta indicate the orientation of substituents around a cyclic structure or at ring fusion sites in cyclic molecules like. Epimers are stereoisomers that differ in their configuration at. The stereochemical designators α and β distinguish between: Web specifically, the α designator is used for substituents that are located on the same side of the. Epimers at the anomeric carbon. Web the stereochemical designators alpha and beta indicate the orientation of substituents around a cyclic structure or at ring fusion sites in cyclic molecules like. The stereochemical designators α and β distinguish between: Diastereomers have different physical properties (melting points,. Incorporate the e,z designation into the iupac name of a given alkene. Web specifically, the α designator is used for substituents that are located on the same side of the molecule's carbon atom, while the β designator is used for. Enantiomers at an anomeric carbon atom. Epimers at the anomeric carbon. Diastereomers have different physical properties (melting points,. Enantiomers at an anomeric carbon atom c. Web the study of stereochemistry focuses on the relationships between stereoisomers, which by definition have the same molecular formula and sequence of bonded atoms. The stereochemical designators alpha and beta distinguish between: Web the question says the stereochemical designators alpha and beta distinguish between the answer is epimers at the anomeric carbon. Isn't this supposed to be anomers? Web use the e,z designation to describe the geometry of a given alkene structure. Web study with quizlet and memorize flashcards containing terms like the stereochemical designators α and β distinguish between:, structures of nucleotides, is atp positively. Web study with quizlet and memorize flashcards containing terms like the stereochemical designators α and β distinguish between which stereoisomers?, how does na+ flow. Several various classes and properties of isomers relevant to the mcat will be examined here. Web chemists need a convenient way to distinguish one stereoisomer from another. Enantiomers at an epimeric carbon atom. Web the stereochemical designators α and β distinguish between: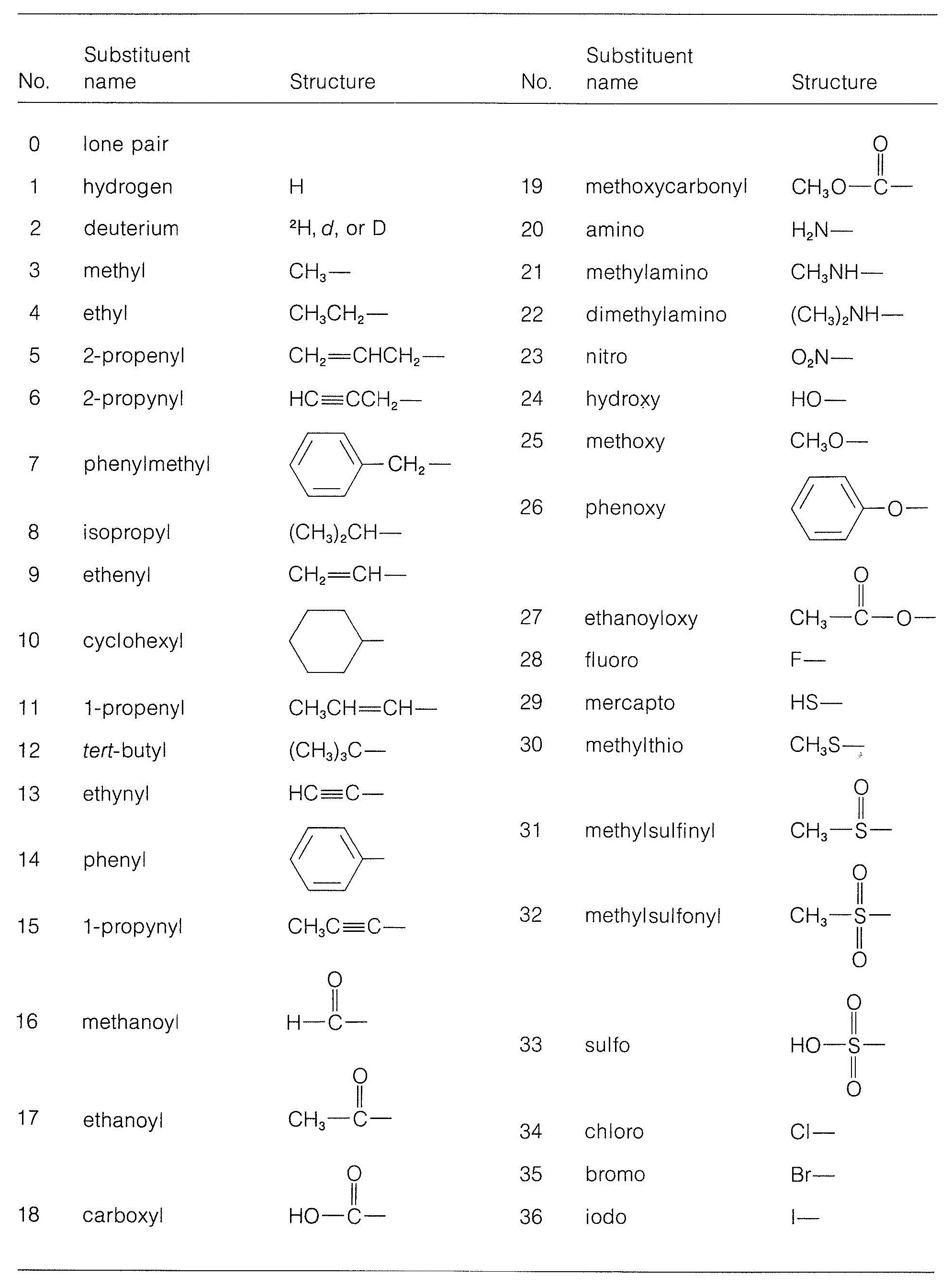
19.6 The R,S Convention for Designating Stereochemical Configurations
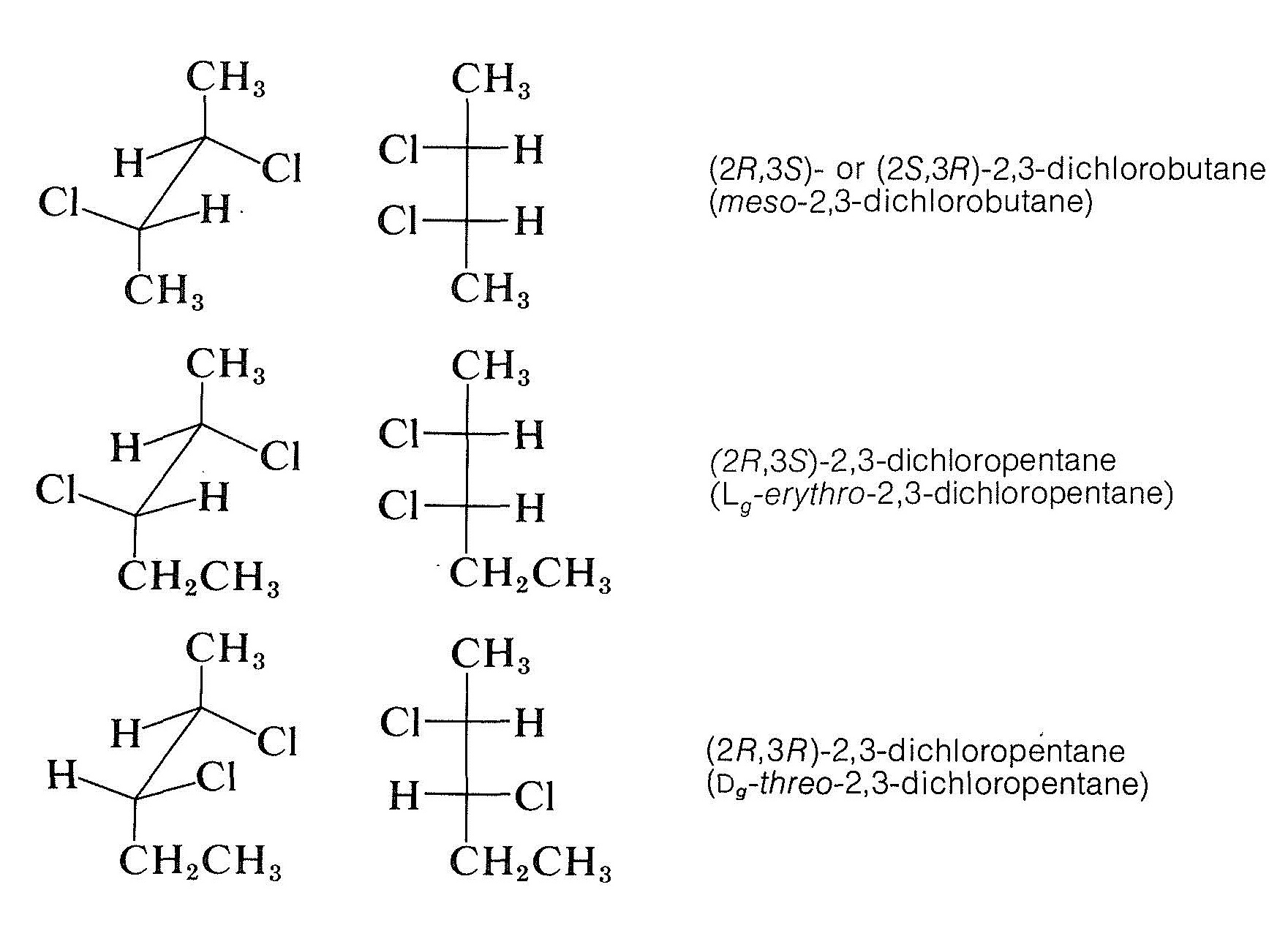
19.6 The R,S Convention for Designating Stereochemical Configurations
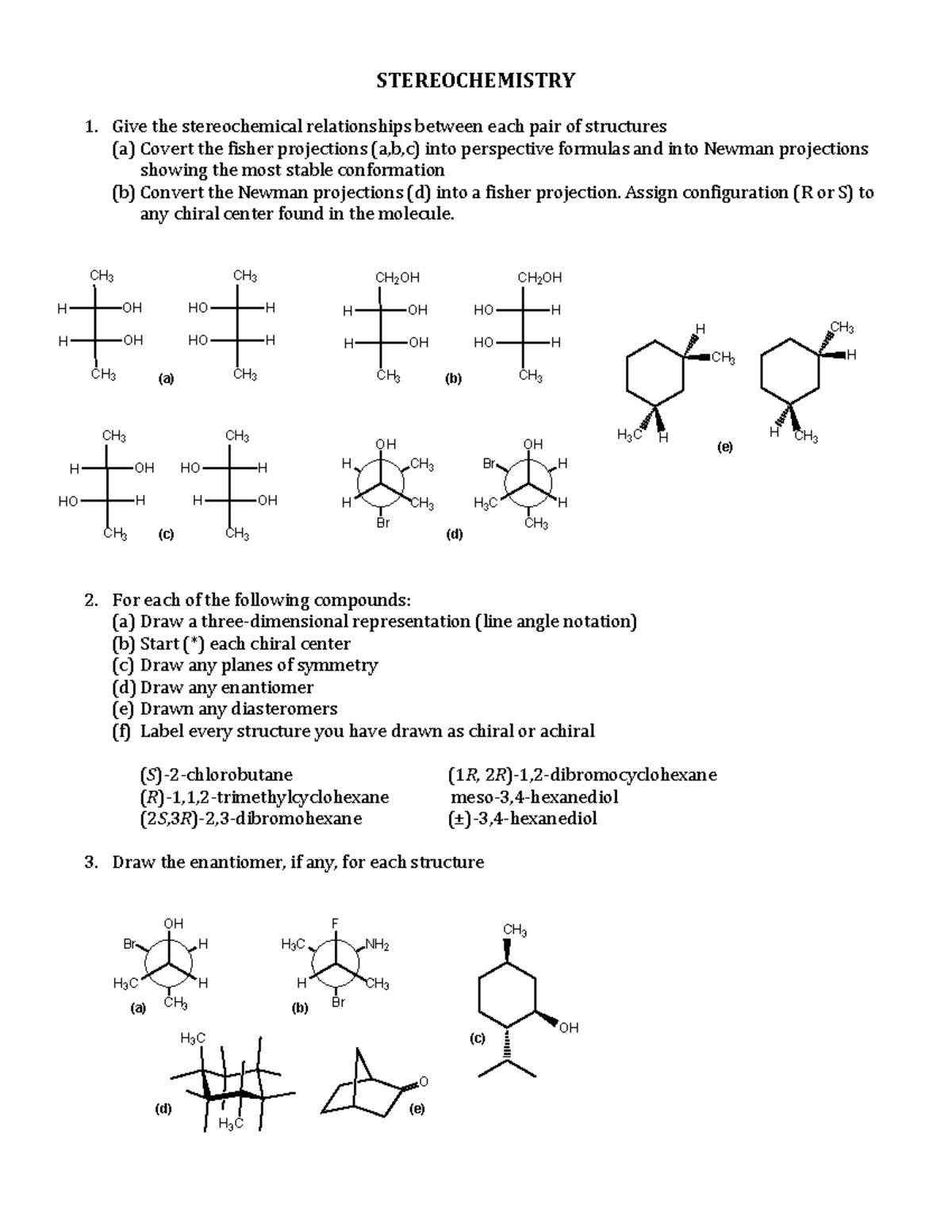
Chapter 3 Stereochemistry STEREOCHEMISTRY 1. Give the stereochemical

Introduction to the R,S Stereochemical Designations Used in Chemical

Stereochemical structures of substrates. Download Scientific Diagram

Stereochemistry notes
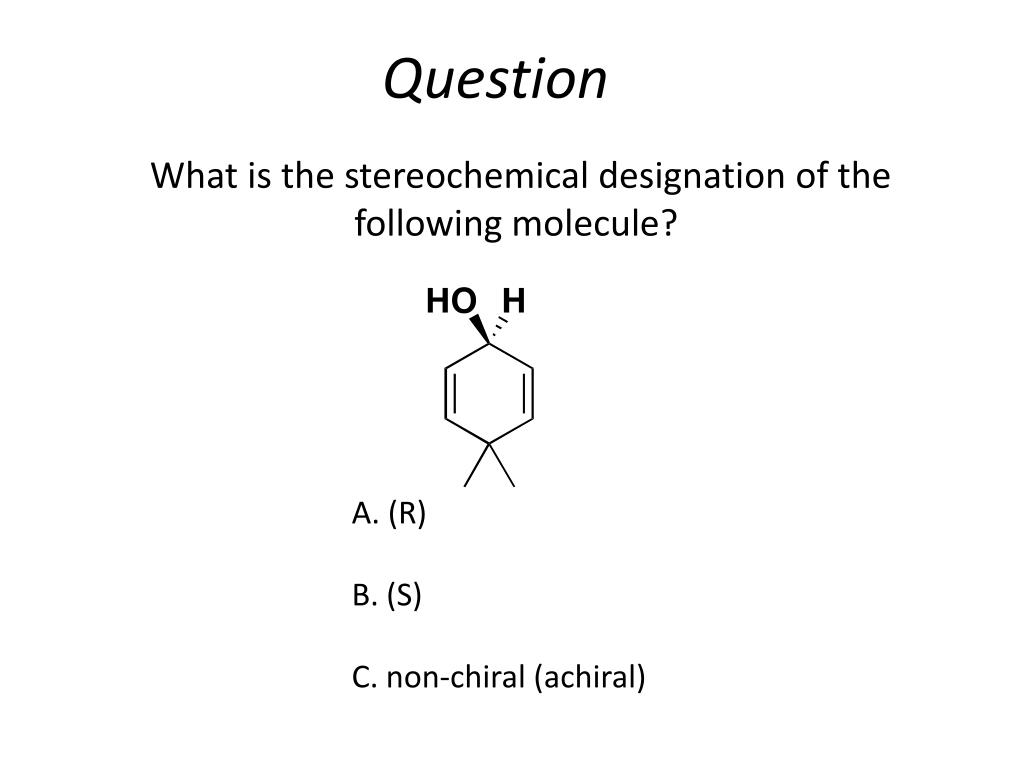
PPT Stereochemistry PowerPoint Presentation, free download ID2730590
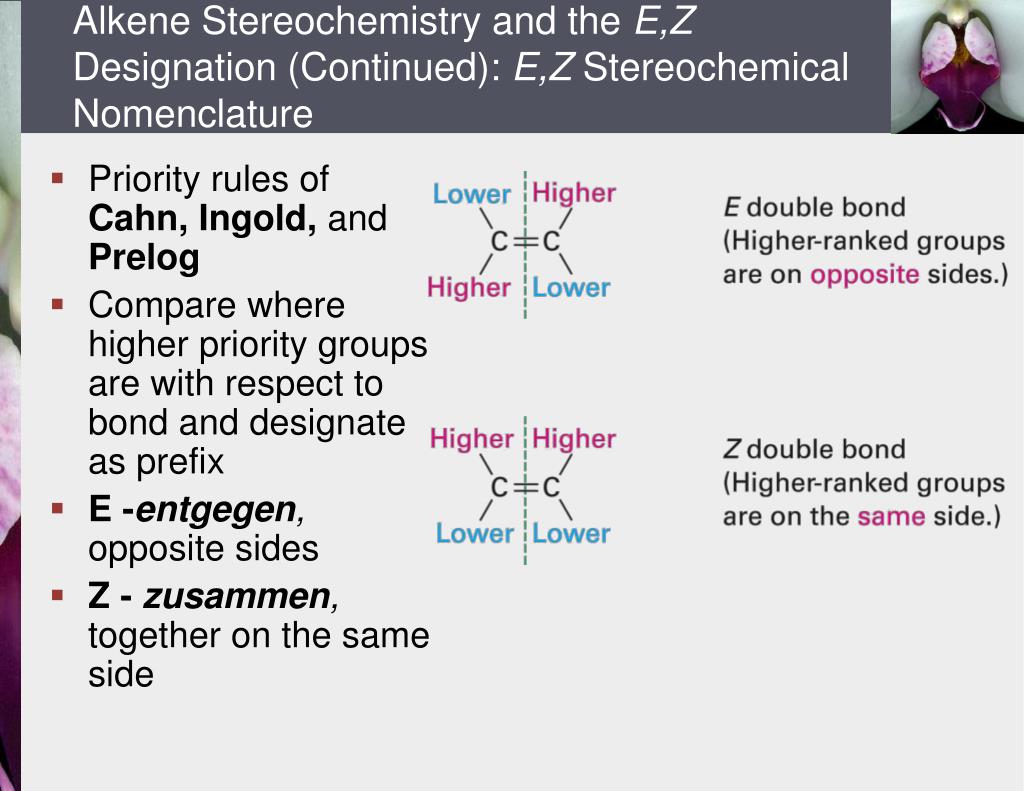
PPT Chapter 7 Alkenes Structure and Reactivity PowerPoint

PPT Stereochemistry PowerPoint Presentation ID2202919

PPT 5. Stereochemistry PowerPoint Presentation, free download ID
Web Diastereomers Are Stereoisomers With Two Or More Chiral Centers That Are Not Enantiomers.
Which Ideal Solution Exhibits The Greatest Osmotic Pressure?
Web The Stereochemical Designators Α And Β Distinguish Between Epimers At An Anomeric Carbon Atom.
Web The Stereochemical Designators Alpha And Beta Indicate The Orientation Of Substituents Around A Cyclic Structure Or At Ring Fusion Sites In Cyclic Molecules Like.
Related Post: