Spontaneity Chart
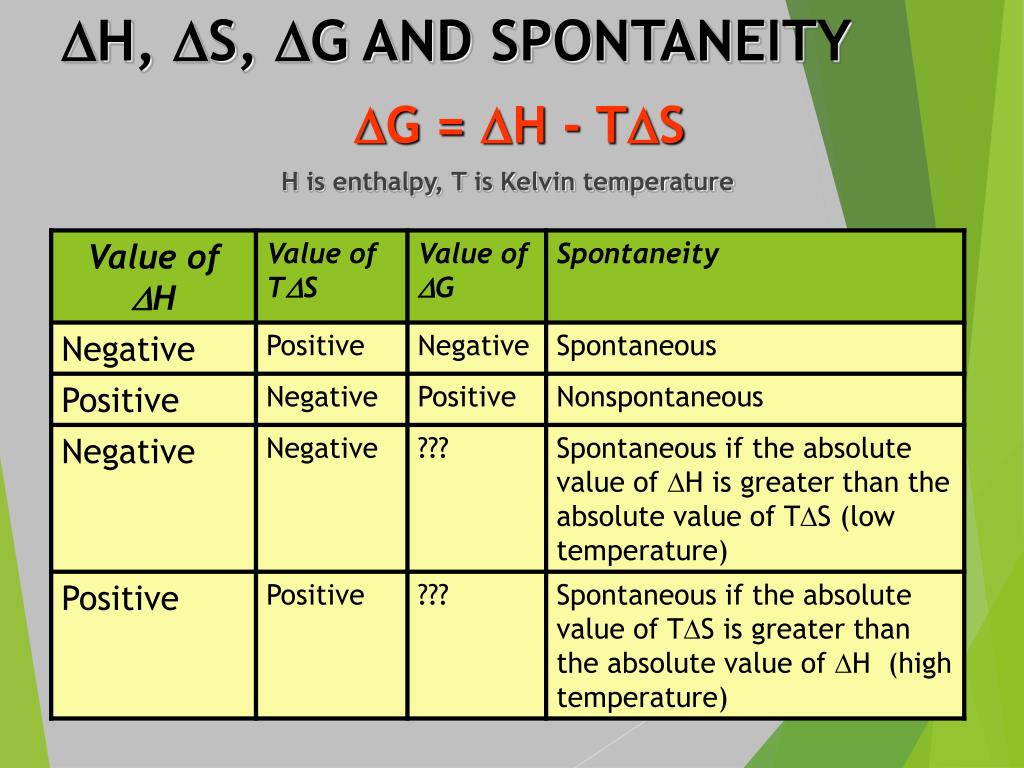
Spontaneity Chart - Negative δg indicates that the reaction is exergonic and spontaneous. Web a spontaneous reaction could take seconds to happen, but it could also take days, years, or even longer. Where h is enthalpy, t. It tells you whether a given reaction will go or not. Web when a process occurs at constant temperature t and pressure p , we can rearrange the second law of thermodynamics and define a new quantity known as gibbs free energy: Because all three criteria are assessing the same thing—the spontaneity of the process—it would be most surprising indeed if they were not related. The change in enthalpy and change in entropy of a reaction are the driving forces behind all chemical reactions. In other terms, a spontaneous process is one that may happen without any intervention. Web the criterion for predicting spontaneity is based on ( δg ), the change in g, at constant temperature and pressure. How do we measure it? Web the free energy change is therefore a reliable indicator of the spontaneity of a process, being directly related to the previously identified spontaneity indicator, δs univ. Web gibbs free energy is probably the most important function in chemistry. Define gibbs free energy, and describe its relation to spontaneity. Where h is enthalpy, t. How do we measure it? In this video i want to do something a little bit more rigorous and actually, i guess you could say derive the formula. Web the change in gibbs free energy associated with a chemical reaction is a useful indicator of whether the reaction will proceed spontaneously. The temperature can be the deciding factor in spontaneity when the enthalpy and entropy. Web when a process occurs at constant temperature t and pressure p , we can rearrange the second law of thermodynamics and define a new quantity known as gibbs free energy: Web the criterion for predicting spontaneity is based on ( δg ), the change in g, at constant temperature and pressure. Web 15 thermochemistry ii spontaneity, entropy and gibbs. Web i've hopefully given you a bit of a gut feeling behind where the formula of gibbs free energy comes from. Where h is enthalpy, t. Web table 18.6.1 summarizes these criteria and their relative values for spontaneous, nonspontaneous, and equilibrium processes. To gain an understanding of the relationship between spontaneity, free energy, and temperature. Reaction that occurs without external. Define gibbs free energy, and describe its relation to spontaneity. Table 16.3 summarizes the relation between the spontaneity of a process and the arithmetic signs of these indicators. To be able to calculate the temperature at which a process is at equilibrium under standard conditions. It is defined by the gibbs equation: We’ve seen from experience that some chemical (and. Web table 18.6.1 summarizes these criteria and their relative values for spontaneous, nonspontaneous, and equilibrium processes. Where h is enthalpy, t. Negative δg indicates that the reaction is exergonic and spontaneous. What is the driving force that creates spontaneity? The relationship between δs univ and δg sys was described in. We’ve seen from experience that some chemical (and other) processes are spontaneous while others are not. What is the driving force that creates spontaneity? Reaction that requires the input of additional action to proceed. Web the temperature plays an important role in determining the gibbs free energy and spontaneity of a reaction. Calculate free energy change for a process using. It tells you whether a given reaction will go or not. Web table 18.6.1 summarizes these criteria and their relative values for spontaneous, nonspontaneous, and equilibrium processes. Web gibbs free energy is a conceptual model for determining the spontaneity of a reaction. The temperature can be the deciding factor in spontaneity when the enthalpy and entropy terms have opposite signs:. Web the temperature plays an important role in determining the gibbs free energy and spontaneity of a reaction. Web a spontaneous reaction could take seconds to happen, but it could also take days, years, or even longer. We’ve seen from experience that some chemical (and other) processes are spontaneous while others are not. Web what is spontaneity? To gain an. Web table 18.6.1 summarizes these criteria and their relative values for spontaneous, nonspontaneous, and equilibrium processes. Gibbs free energy = g = h − ts. Web when a process occurs at constant temperature t and pressure p , we can rearrange the second law of thermodynamics and define a new quantity known as gibbs free energy: Web the temperature plays. Negative δg indicates that the reaction is exergonic and spontaneous. A spontaneous process is one that can occur on its own or as a result of some kind of initiation under certain conditions. Generally, all reactions want to go to a lower energy state, thus a negative change is favored. Since the change in free energy is equal to the maximum useful work which can be accomplished by the reaction. Web gibbs free energy is probably the most important function in chemistry. If it won't go, it tells you how much work you must put into the reaction to get it to go. Reaction that occurs without external activity. Web the criterion for predicting spontaneity is based on ( δg ), the change in g, at constant temperature and pressure. To be able to calculate the temperature at which a process is at equilibrium under standard conditions. Web chemical and physical processes have a natural tendency to occur in one direction under certain conditions. Because all three criteria are assessing the same thing—the spontaneity of the process—it would be most surprising indeed if they were not related. Web the temperature plays an important role in determining the gibbs free energy and spontaneity of a reaction. The relationship between δs univ and δg sys was described in. It tells you whether a given reaction will go or not. If it will go, it tells you how much. In this video i want to do something a little bit more rigorous and actually, i guess you could say derive the formula.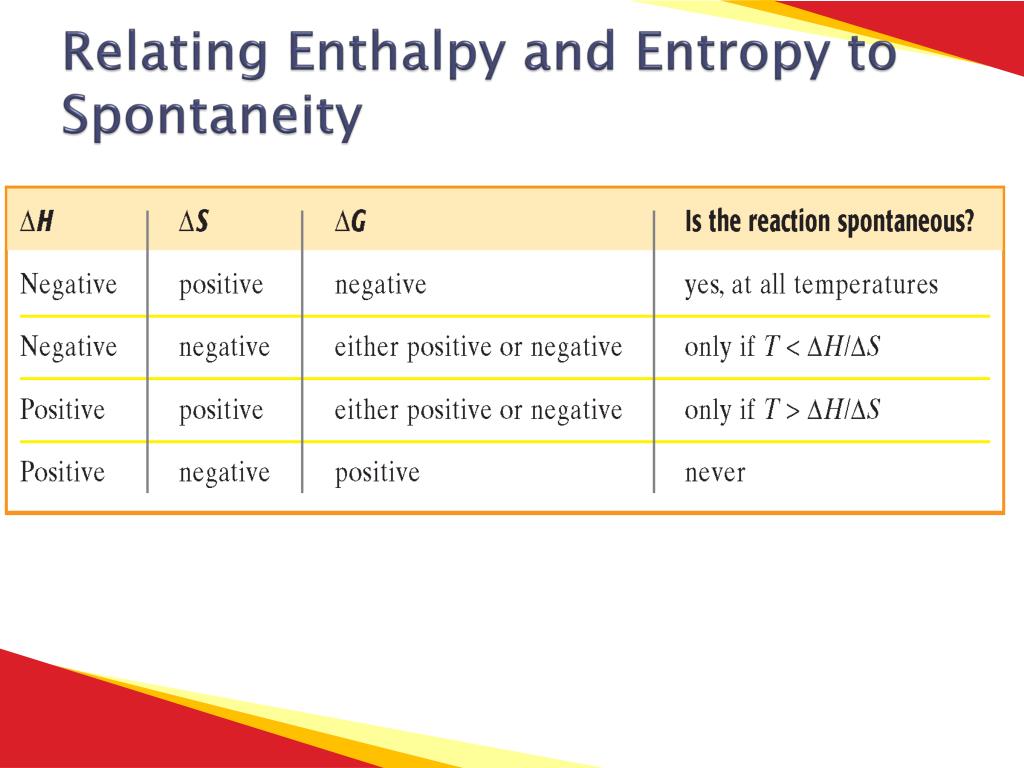
PPT Chapter 16 Reaction Energy PowerPoint Presentation, free download
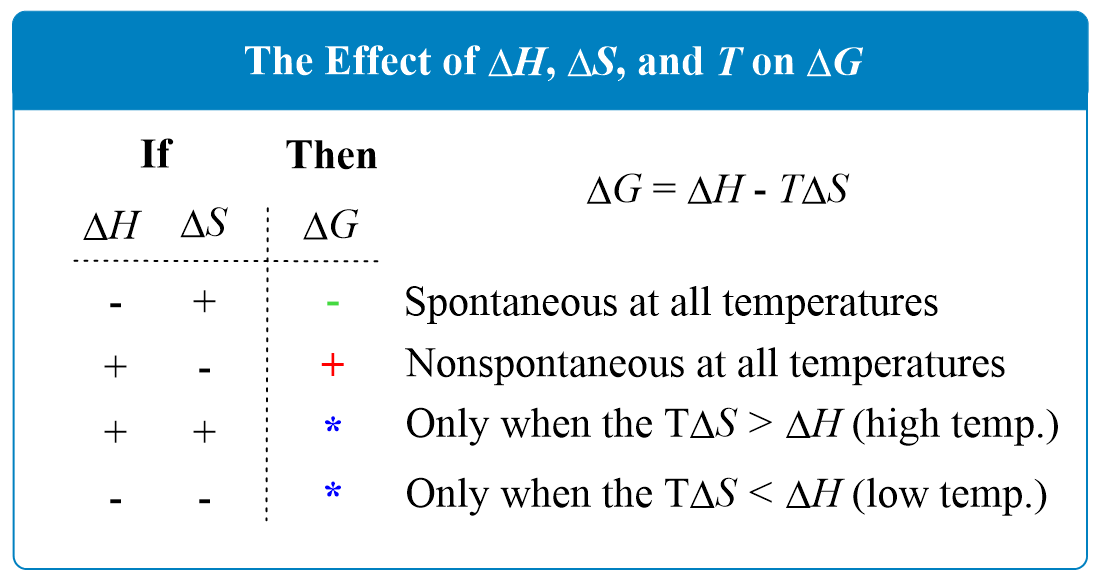
The Effect of 𝚫H, 𝚫S, and T on 𝚫G Spontaneity Chemistry Steps
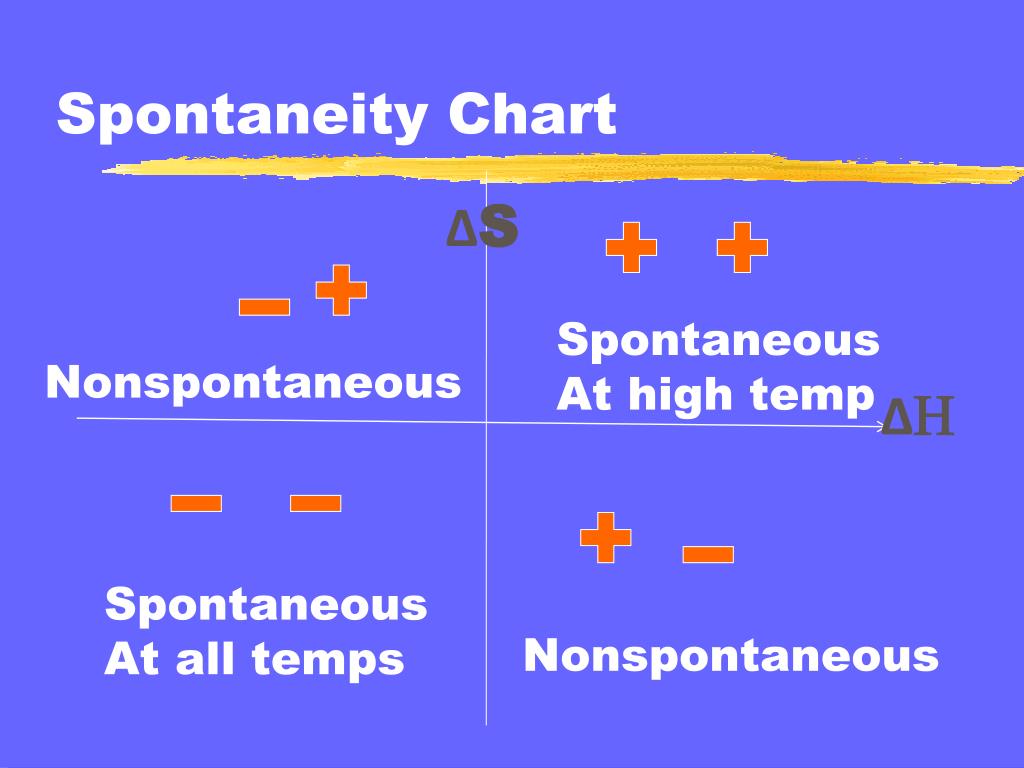
PPT Spontaneity PowerPoint Presentation, free download ID6810823
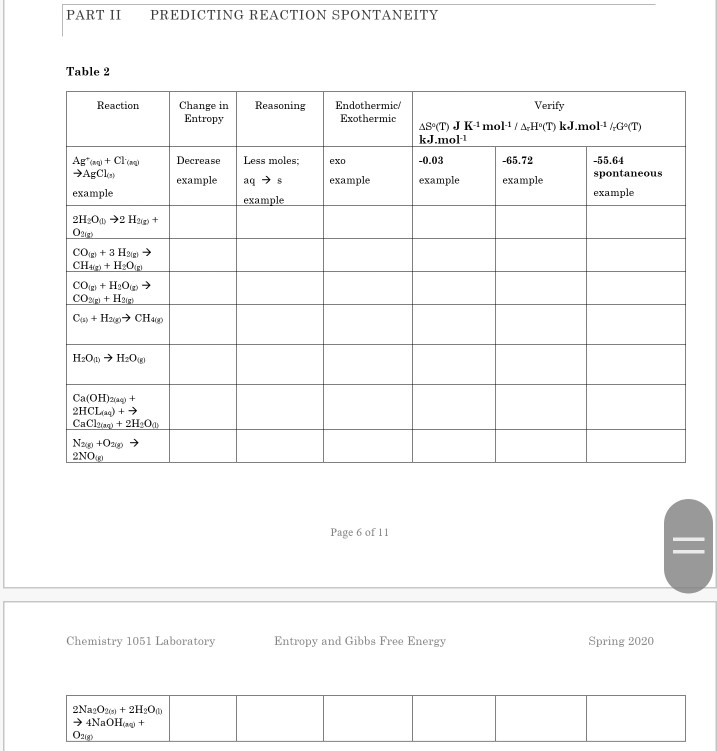
Solved PART II PREDICTING REACTION SPONTANEITY Table 2
Spontaneity, Entropy, and Free Energy Presentation Chemistry
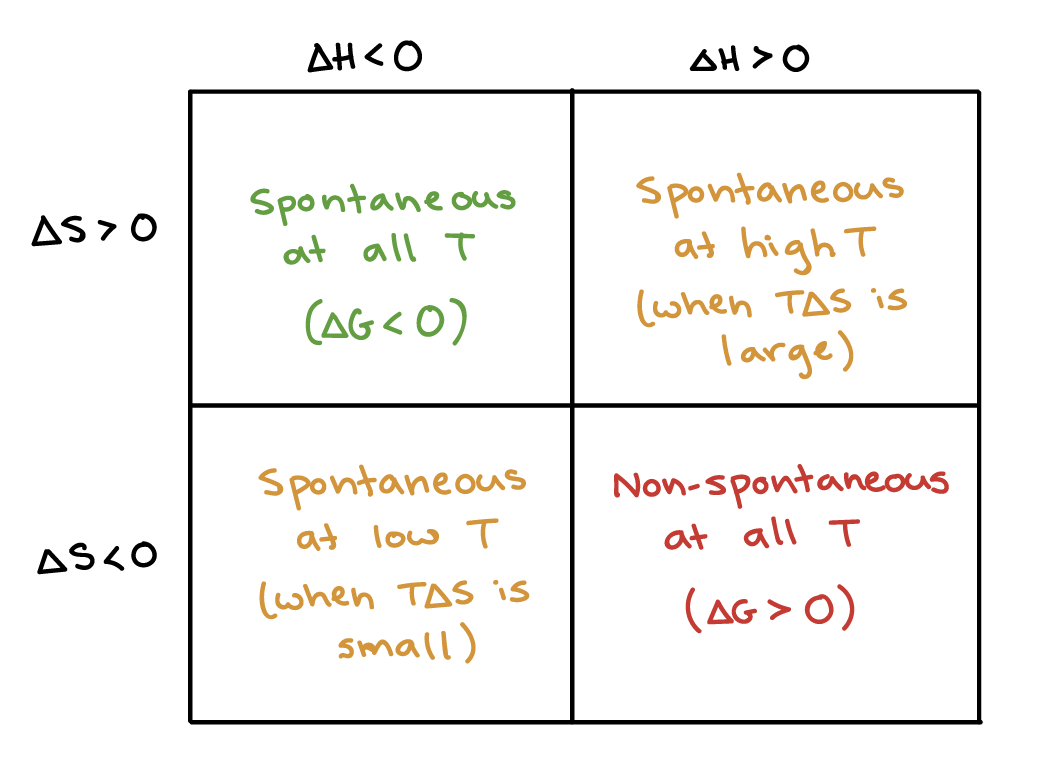
How will temperature affect the spontaneity of a reaction with positive
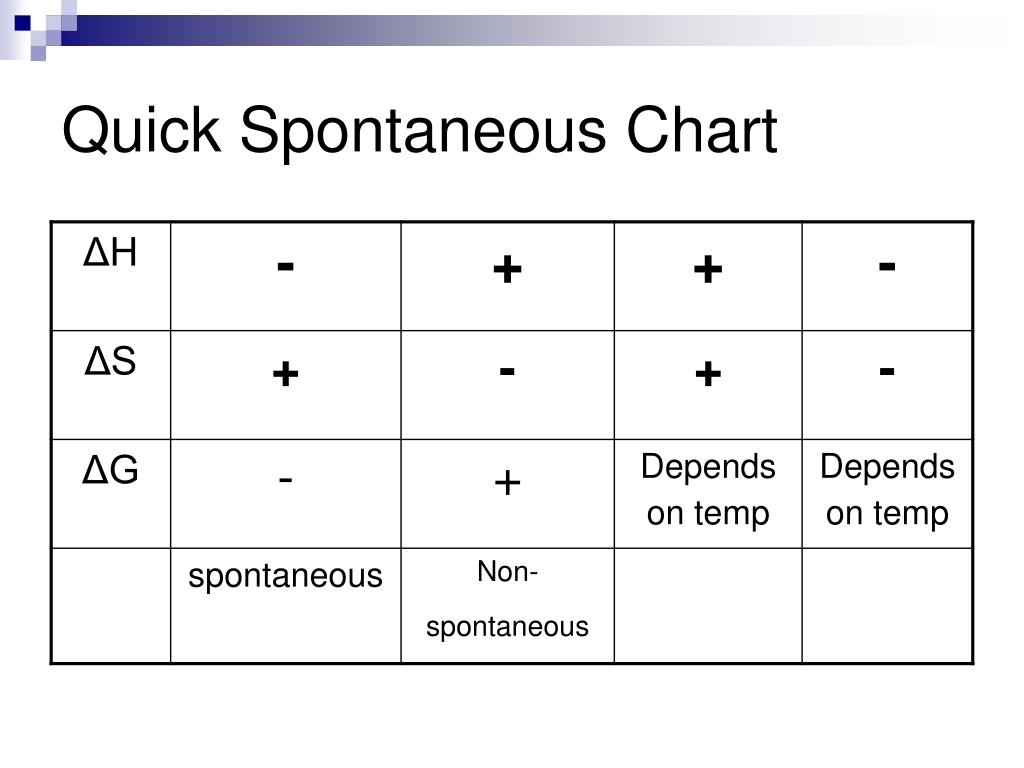
PPT Ch. 10.4 and 11 Thermodynamics PowerPoint Presentation, free
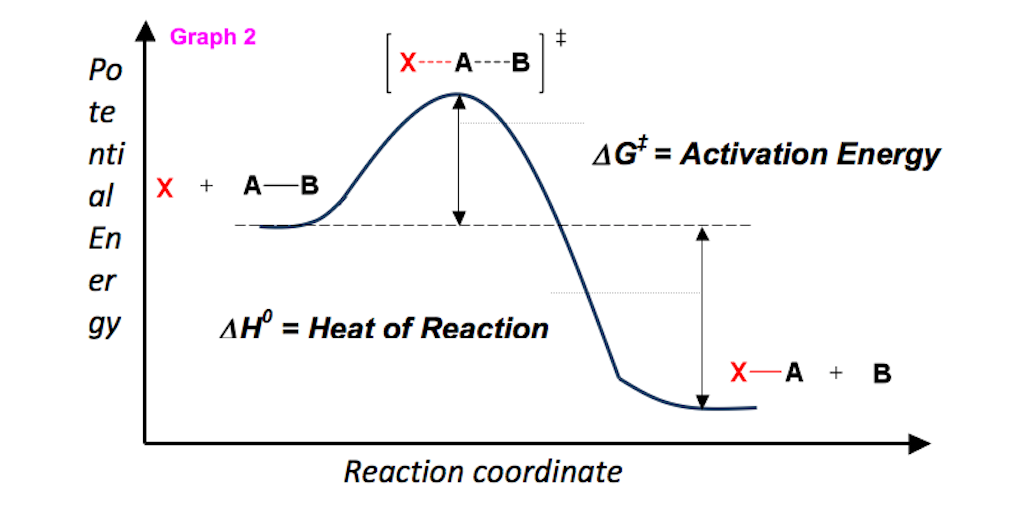
Energy Diagram Module Series Part Two Gibbs Free Energy and Spontaneity
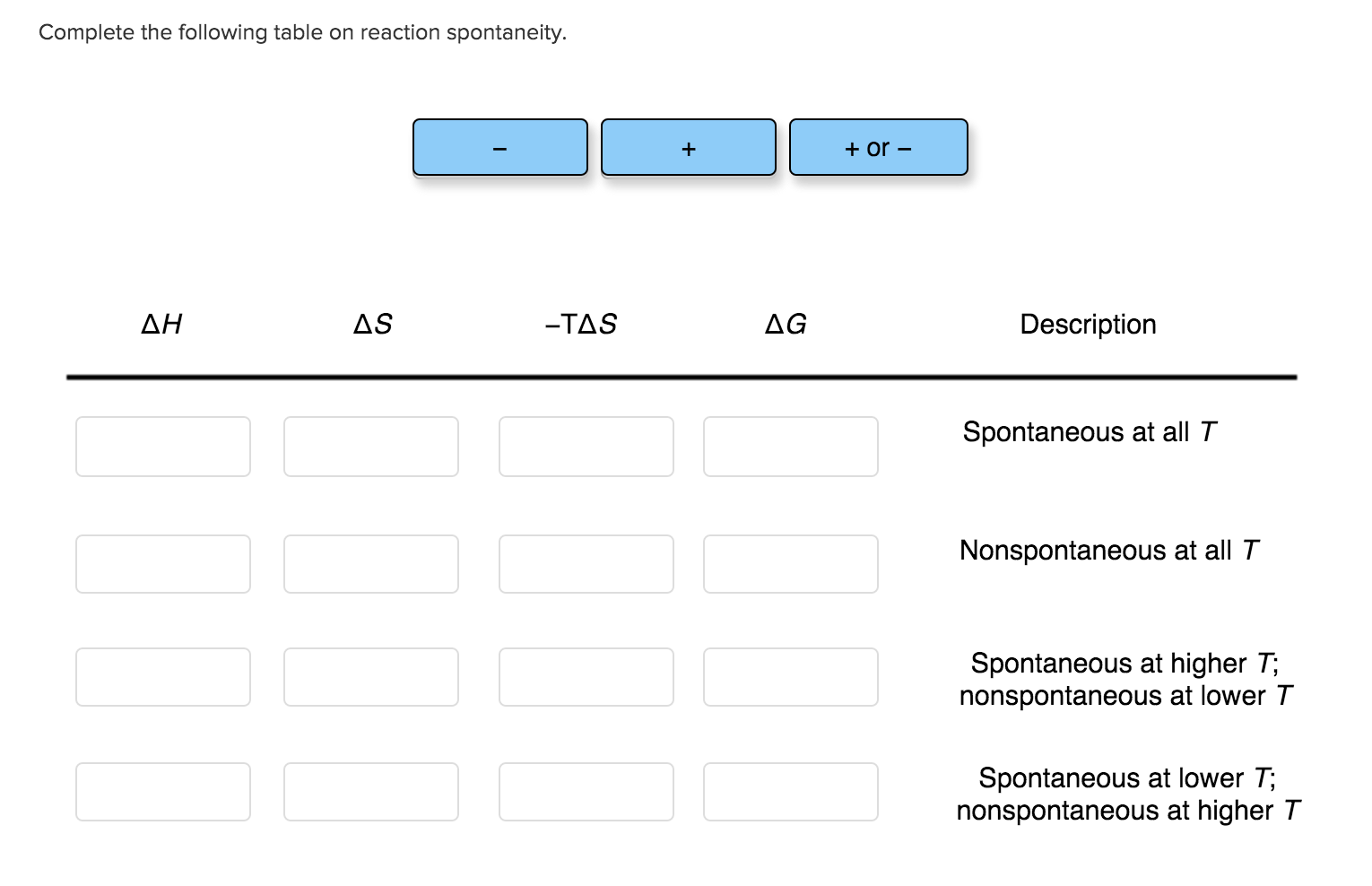
Solved Complete the following table on reaction spontaneity.
PPT Spontaneity, Entropy and Free Energy PowerPoint Presentation
Web Free Energy And Free Energy Change—The Gibbs Free Energy, G, Is Used To Describe The Spontaneity Of A Process.
Web How Does Knowing The Spontaneity Of A Reaction Help A Scientist?
Reaction That Requires The Input Of Additional Action To Proceed.
Web I've Hopefully Given You A Bit Of A Gut Feeling Behind Where The Formula Of Gibbs Free Energy Comes From.
Related Post:
.PNG)