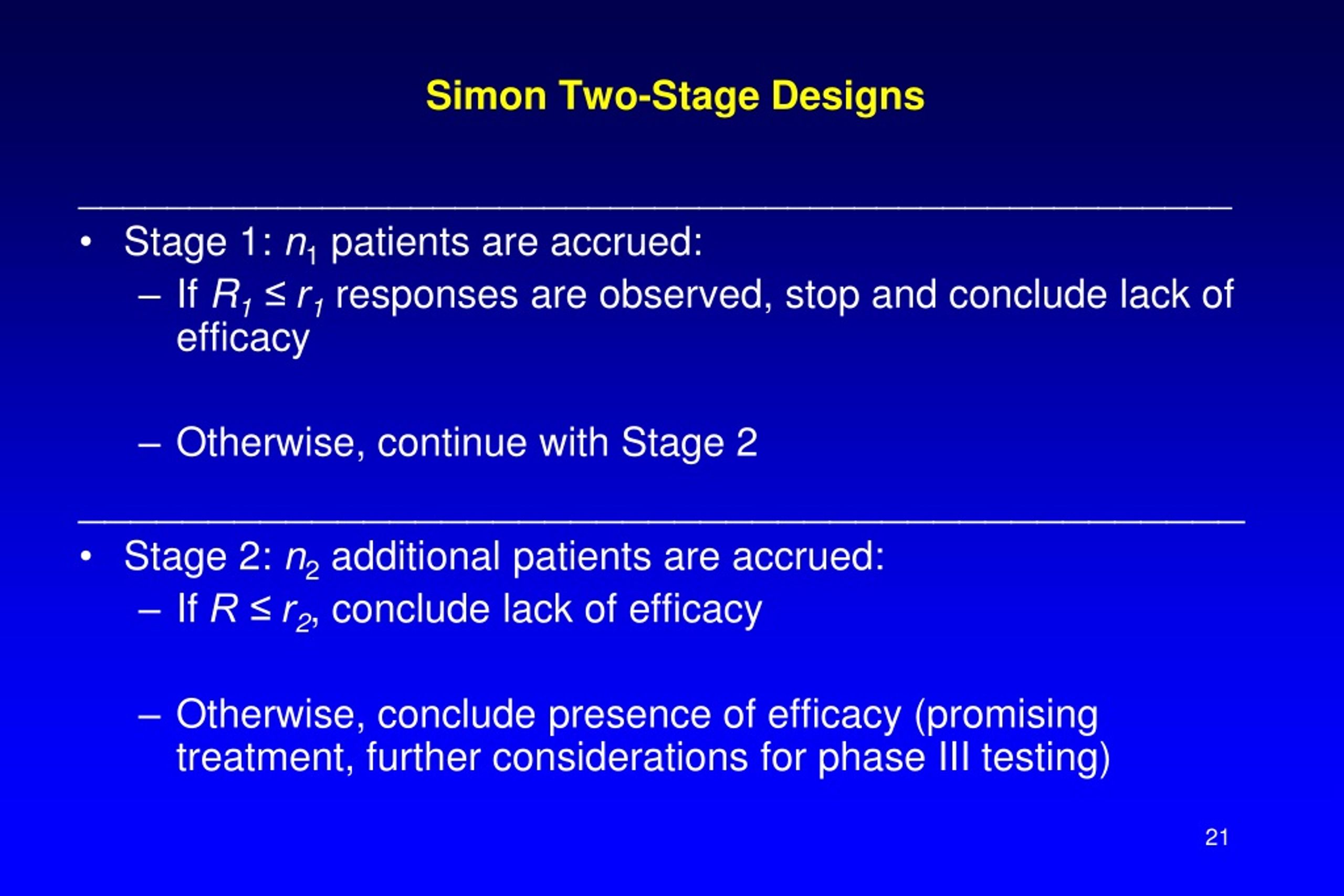
Simon Two Stage Design
Simon Two Stage Design - A study proceeds to the second stage unless the null hypothesis, that the. Jung et al., 2004) for phase ii single arm clinical trials with relaxed probability of stopping for futility. (2004) for phase ii single arm. In such trials, the second stage sample. Web their adaptive design uses results from a single interim analysis to decide whether to enrich the study population with a subgroup or not; Web simon r (1989). The maximum sample size and the. These methods rely on statistical testing and thus allow controlling the type i and ii error rates,. Web in this paper, we review and extend a biomarker stratified simon two‐stage design proposed by jones and holmgren (jh) 20 in the context of phase ii cancer. (n 1, n, r 1, r), where n 1 and n are the first stage and the maximum total sample size,. Web simon's two stage design. Jung et al., 2004) for phase ii single arm clinical trials with relaxed probability of stopping for futility. In such trials, the second stage sample. Web simon r (1989). Web their adaptive design uses results from a single interim analysis to decide whether to enrich the study population with a subgroup or not; Web simon's two stage design. Web simon r (1989). (2004) for phase ii single arm. In such trials, the second stage sample. Web in this paper, we review and extend a biomarker stratified simon two‐stage design proposed by jones and holmgren (jh) 20 in the context of phase ii cancer. The optimal design is a. A study proceeds to the second stage unless the null hypothesis, that the. Web in this paper, we review and extend a biomarker stratified simon two‐stage design proposed by jones and holmgren (jh) 20 in the context of phase ii cancer. (n 1, n, r 1, r), where n 1 and n are the first. Jung et al., 2004) for phase ii single arm clinical trials with relaxed probability of stopping for futility. The maximum sample size and the. Web simon's two stage design. In such trials, the second stage sample. (n 1, n, r 1, r), where n 1 and n are the first stage and the maximum total sample size,. Web simon's two stage design. A study proceeds to the second stage unless the null hypothesis, that the. The optimal design is a. (n 1, n, r 1, r), where n 1 and n are the first stage and the maximum total sample size,. These methods rely on statistical testing and thus allow controlling the type i and ii error. Jung et al., 2004) for phase ii single arm clinical trials with relaxed probability of stopping for futility. Web in this paper, we review and extend a biomarker stratified simon two‐stage design proposed by jones and holmgren (jh) 20 in the context of phase ii cancer. Web simon r (1989). Web simon's two stage design. (2004) for phase ii single. Jung et al., 2004) for phase ii single arm clinical trials with relaxed probability of stopping for futility. (2004) for phase ii single arm. Web simon r (1989). In such trials, the second stage sample. The maximum sample size and the. Web in this paper, we review and extend a biomarker stratified simon two‐stage design proposed by jones and holmgren (jh) 20 in the context of phase ii cancer. (n 1, n, r 1, r), where n 1 and n are the first stage and the maximum total sample size,. The maximum sample size and the. Web simon's two stage design.. These methods rely on statistical testing and thus allow controlling the type i and ii error rates,. Web in this paper, we review and extend a biomarker stratified simon two‐stage design proposed by jones and holmgren (jh) 20 in the context of phase ii cancer. The optimal design is a. Jung et al., 2004) for phase ii single arm clinical. In such trials, the second stage sample. (n 1, n, r 1, r), where n 1 and n are the first stage and the maximum total sample size,. Web simon's two stage design. Web their adaptive design uses results from a single interim analysis to decide whether to enrich the study population with a subgroup or not; A study proceeds. Web simon's two stage design. (n 1, n, r 1, r), where n 1 and n are the first stage and the maximum total sample size,. Web simon r (1989). (2004) for phase ii single arm. These methods rely on statistical testing and thus allow controlling the type i and ii error rates,. Web their adaptive design uses results from a single interim analysis to decide whether to enrich the study population with a subgroup or not; The optimal design is a. In such trials, the second stage sample. Web in this paper, we review and extend a biomarker stratified simon two‐stage design proposed by jones and holmgren (jh) 20 in the context of phase ii cancer.
Flowchart for Simon’s twostage design Download Scientific Diagram
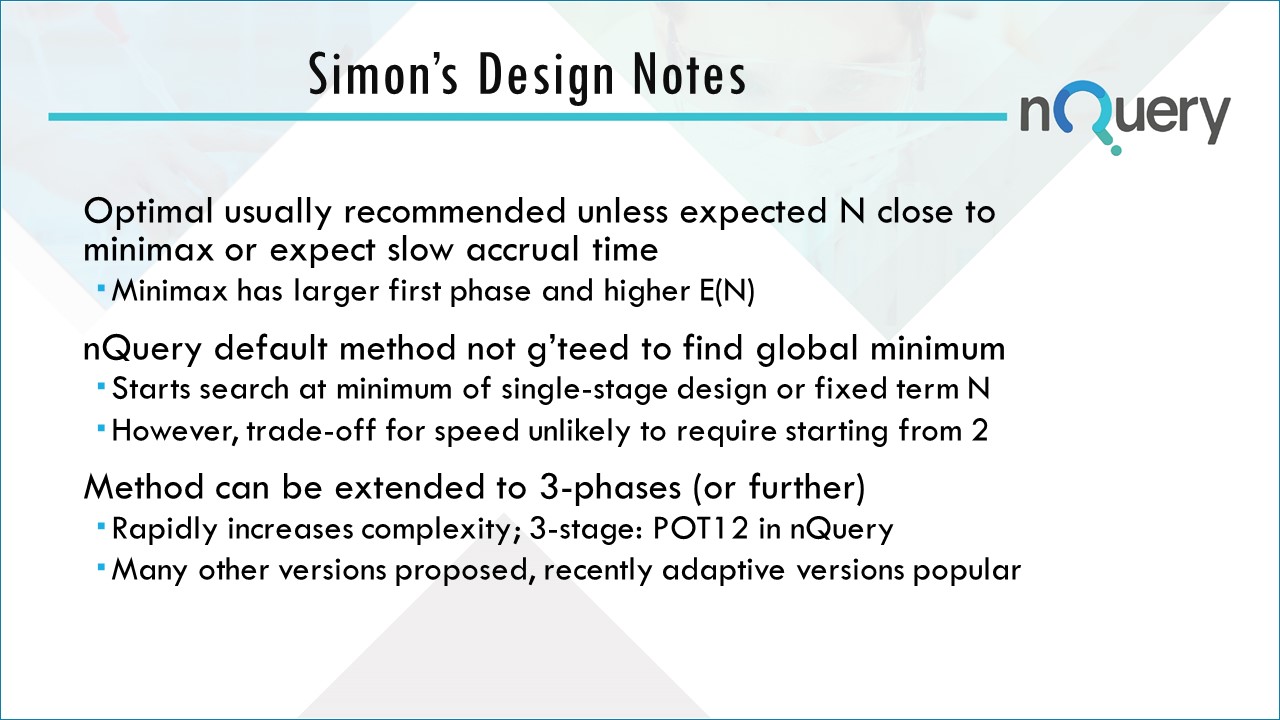
Simon's Two Stage Design

Simon's Two Stage Design

The Simon twostage optimal design algorithm of the study. Download
Simon' s twostage design. TRG, tumour regression grade. Download
PPT Designs for Phase II Clinical Trials PowerPoint Presentation
 - Sample Size Example_Page_7_Image_0002.jpeg?width=1599&name=Two Stage Phase II (Simons Design) - Sample Size Example_Page_7_Image_0002.jpeg)
Hypothesis Testing Example Simon's two stage design

Adaptive parallel Simon twostage design. “R” refers to randomization

The Simon twostage optimal design algorithm of the study. Download
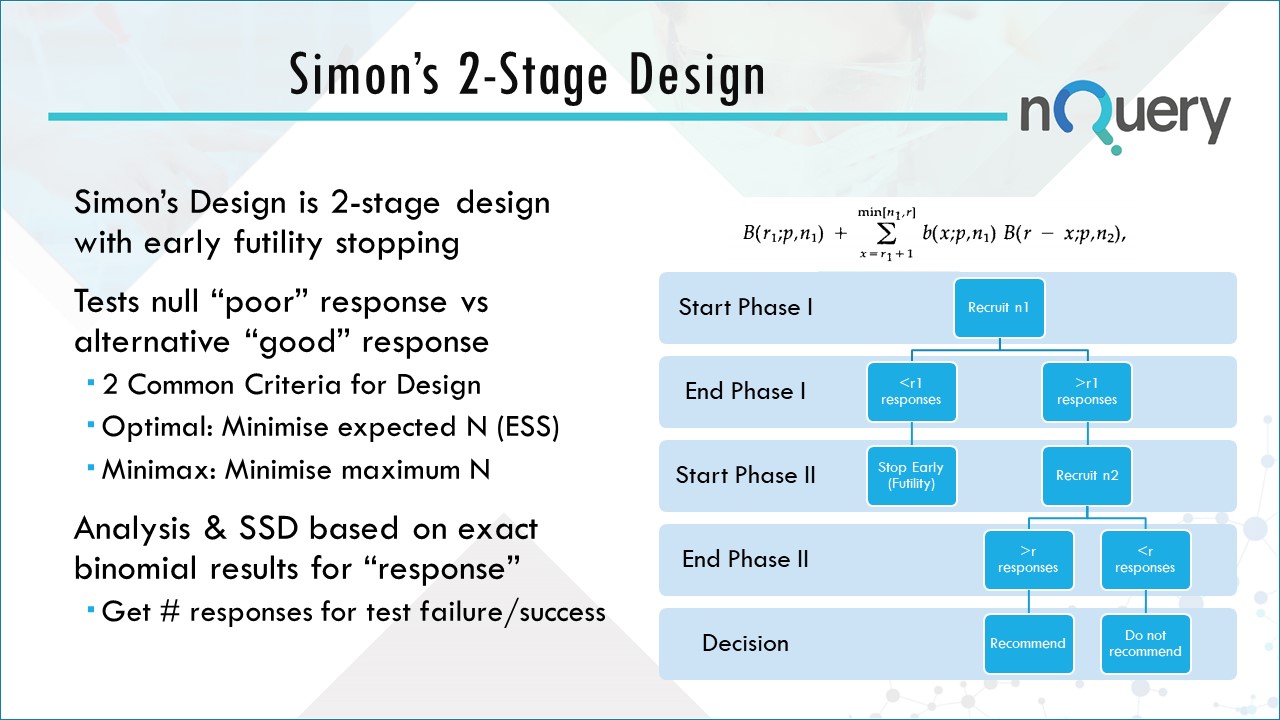
Simon's Two Stage Design
A Study Proceeds To The Second Stage Unless The Null Hypothesis, That The.
The Maximum Sample Size And The.
Jung Et Al., 2004) For Phase Ii Single Arm Clinical Trials With Relaxed Probability Of Stopping For Futility.
Related Post: