Second Ionization Energy Chart
Second Ionization Energy Chart - Web an element's first ionization energy is the energy required to remove the outermost, or least bound, electron from a neutral atom of the element. Web for instance, the 1st ionization energy of the element m is a measure of the energy required to remove one electron from one mole of the gaseous ion m while the 2nd ionization energy of the element m is a measure of the energy required to remove one electron from one mole of the gaseous ion m +. Web so without actually providing the ionization energies for all the group 13 elements, they could say that the element has the second highest first ionization energy in its group, which would be aluminum. Likewise, the amount of energy required to remove an electron from a divalent cation in its gaseous state is called the third ionization energy, and so on. Web but if you want to see the ionization energy of all the 118 elements, then visit: (here you will get the first, second and third ionization energy of all the elements in a single chart). On the periodic table, first ionization energy generally increases as you move left to right across a period. Moving down the noble gases group, the ionization energy decreases. Web we can define a first ionization energy (\(i_1\)), a second ionization energy (\(i_2\)), and in general an nth ionization energy (\(i_n\)) according to the following reactions: Web the energy needed to detach one electron is called the first ionization energy, to detach next one the second ionization energy, etc. Web this page explains what second, third, (etc) ionisation energy means, and then looks at patterns in successive ionisation energies for selected elements. Learn the definition, trend on the periodic table, first & second ionization energies, see a chart and much more. Web the second ionization energy ( ie2) is the energy required to remove an electron from a 1+. However, they only have the highest ionization energy in their period because they are the most stable elements. So, by measuring the kinetic energy of the photoelectron ( ke electron ), we can calculate the binding energy ( be ) of the electron in the sample: Web for each atom, the column marked 1 is the first ionization energy to. On the periodic table, first ionization energy generally decreases as you move down a group. (here you will get the first, second and third ionization energy of all the elements in a single chart). Web the amount of energy required to remove an electron from a monovalent cation in its gaseous state is called the second ionization energy. The first. Web you’re right that the noble gases have high ionization energy. Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. Web the second ionization energy ( ie2) is the energy required to remove an electron from a 1+ cation in the gaseous state. Web the second ionization energy is the energy. Web we can define a first ionization energy (\(i_1\)), a second ionization energy (\(i_2\)), and in general an nth ionization energy (\(i_n\)) according to the following reactions: Learn the definition, trend on the periodic table, first & second ionization energies, see a chart and much more. On the periodic table, first ionization energy generally decreases as you move down a. Web you’re right that the noble gases have high ionization energy. So, by measuring the kinetic energy of the photoelectron ( ke electron ), we can calculate the binding energy ( be ) of the electron in the sample: It assumes that you understand about first ionisation energy. Moving down the noble gases group, the ionization energy decreases. The first. Web an element's first ionization energy is the energy required to remove the outermost, or least bound, electron from a neutral atom of the element. Moving down the noble gases group, the ionization energy decreases. On the periodic table, first ionization energy generally decreases as you move down a group. Web the energy required to remove the second most loosely. Web the energy needed to detach one electron is called the first ionization energy, to detach next one the second ionization energy, etc. Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. Web but if you want to see the ionization energy of all the 118 elements, then visit: Moving down. Below are the chemical equations describing the first and second ionization energies: Web you’re right that the noble gases have high ionization energy. Image showing periodicity of the chemical elements for ionization energy: The first ionization energy of the neutral molecule (without charge) is approximately equal to the energy of the highest occupied molecular orbital (homo). Web the second ionization. Web but if you want to see the ionization energy of all the 118 elements, then visit: Web we can define a first ionization energy (\(i_1\)), a second ionization energy (\(i_2\)), and in general an nth ionization energy (\(i_n\)) according to the following reactions: Web for instance, the 1st ionization energy of the element m is a measure of the. We know the energy of the radiation ( h ν ) used to eject the electron. Web you’re right that the noble gases have high ionization energy. Web what is ionization energy? Image showing periodicity of the chemical elements for ionization energy: Ionization energy chart of all the elements. Web the energy required to remove the second most loosely bound electron is called the second ionization energy (ie 2). Web the symbol i1 stands for the first ionization energy (energy required to take away an electron from a neutral atom, where n = 0 ). Web an element's second ionization energy is the energy required to remove the outermost, or least bound, electron from a 1+ ion of the element. This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. Web we can define a first ionization energy (\(i_1\)), a second ionization energy (\(i_2\)), and in general an nth ionization energy (\(i_n\)) according to the following reactions: The first ionization energy of the neutral molecule (without charge) is approximately equal to the energy of the highest occupied molecular orbital (homo). On the periodic table, first ionization energy generally increases as you move left to right across a period. Web this page explains what second, third, (etc) ionisation energy means, and then looks at patterns in successive ionisation energies for selected elements. The first molar ionization energy applies to the neutral atoms. Likewise, the amount of energy required to remove an electron from a divalent cation in its gaseous state is called the third ionization energy, and so on. Web so without actually providing the ionization energies for all the group 13 elements, they could say that the element has the second highest first ionization energy in its group, which would be aluminum.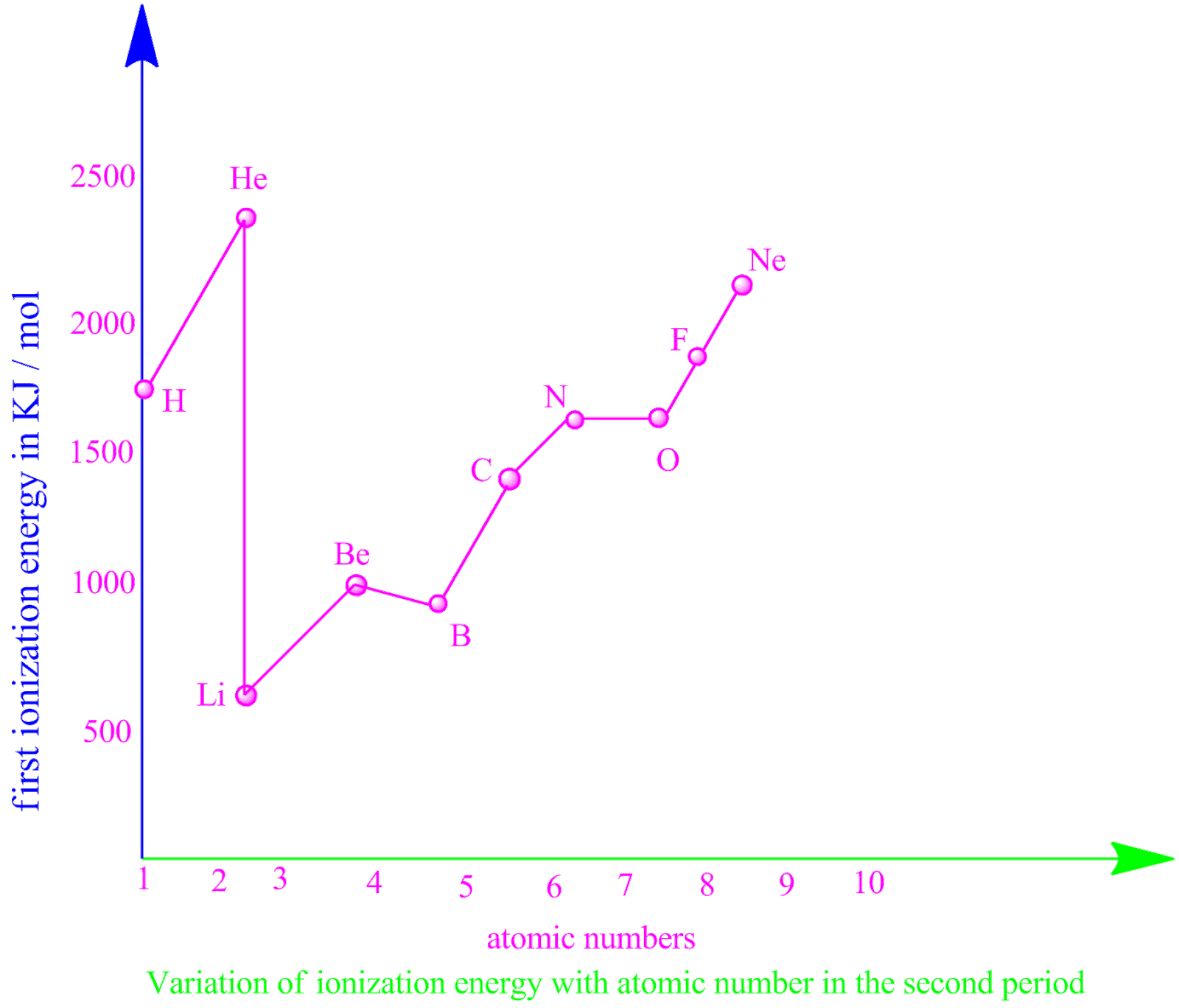
What is ionization energy and why second ionization energy is greater
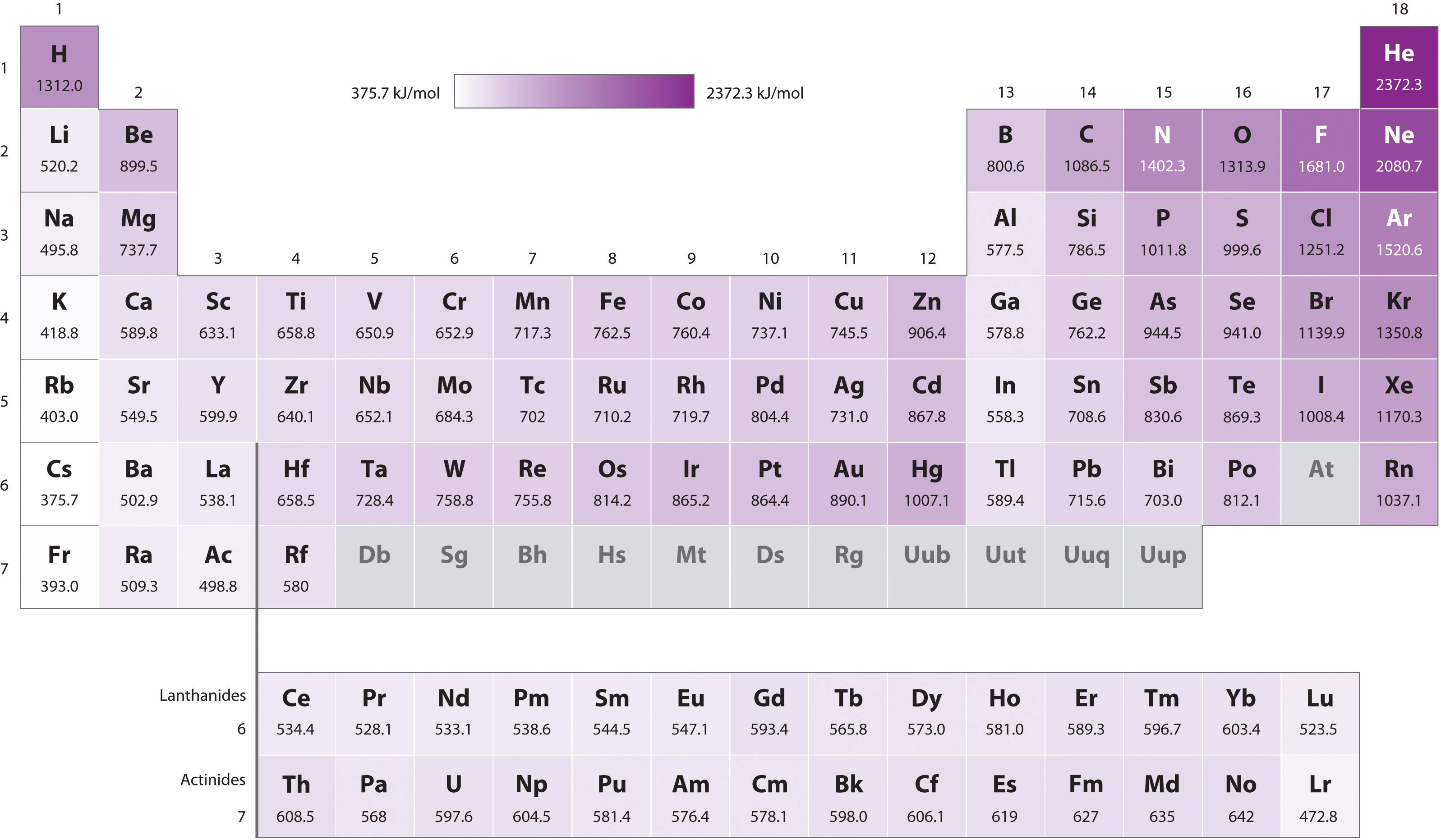
7.4 Ionization Energy Chemistry LibreTexts
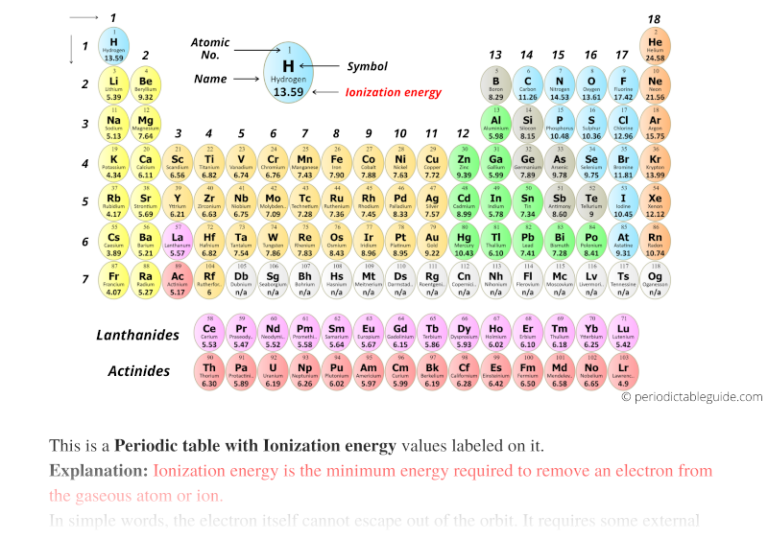
Periodic table f pastorsec
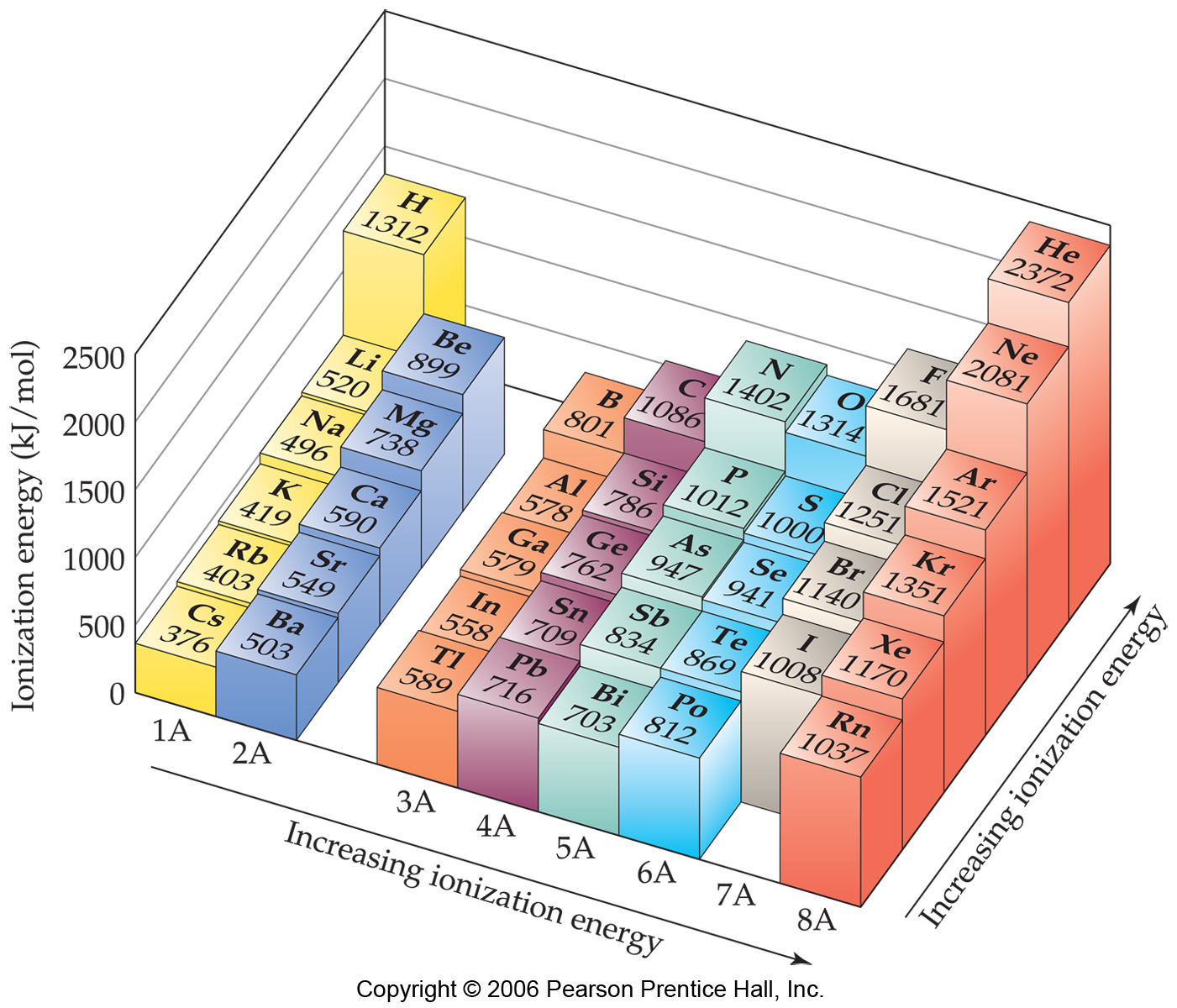
Ionization Energy the amount of energy required to remove an electron
Periodic Trends in Ionization Energy Chemistry Socratic

Second Ionization Energy Periodic Table Trend Review Home Decor

Second Ionization Energy Periodic Table
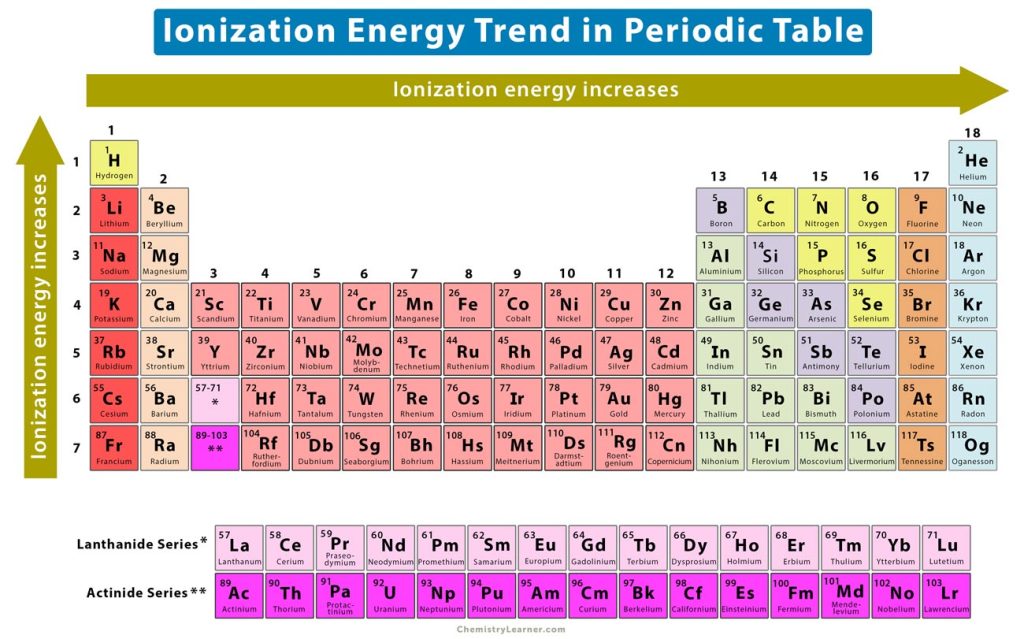
Periodic Trends Definition and Properties
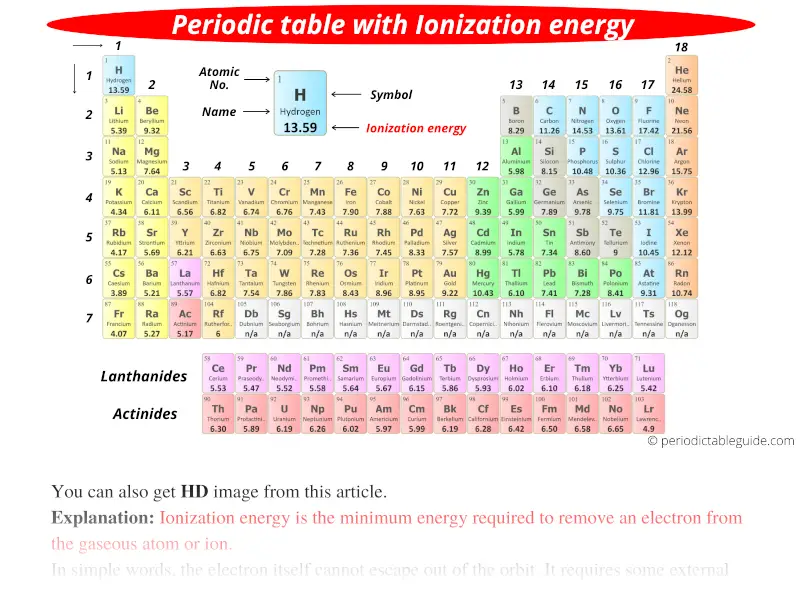
Ionization Energy Table Of Elements Elcho Table
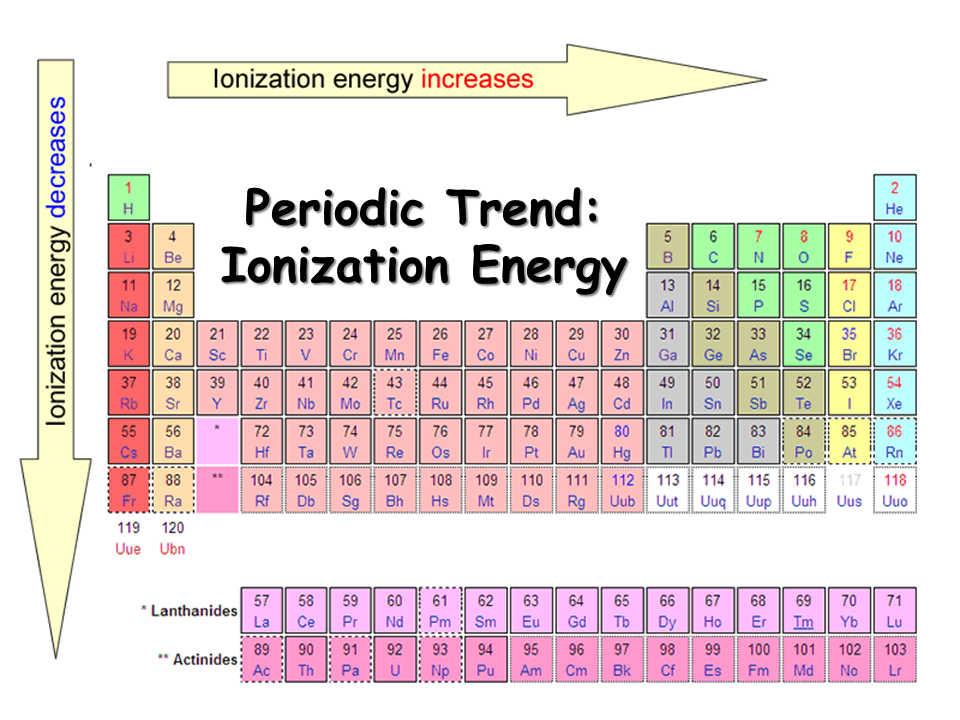
Ionization Enthalpy NEET Lab
Web The 2Nd Ionization Energy Of The Element M Is A Measure Of The Energy Required To Remove One Electron From One Mole Of The Gaseous Ion M +.
Web Explore How Ionization Energy Changes With Atomic Number In The Periodic Table Of Elements Via Interactive Plots.
Web The Energy Needed To Detach One Electron Is Called The First Ionization Energy, To Detach Next One The Second Ionization Energy, Etc.
Web The Amount Of Energy Required To Remove An Electron From A Monovalent Cation In Its Gaseous State Is Called The Second Ionization Energy.
Related Post: