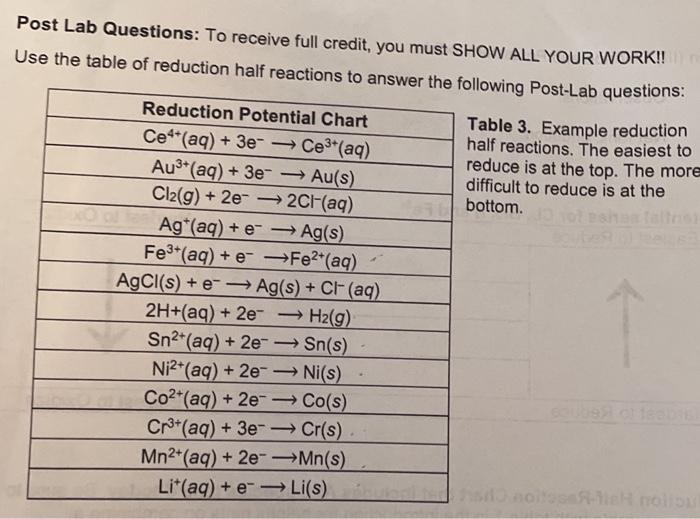
Reduction Half Reaction Chart
Reduction Half Reaction Chart - Use standard reduction potentials to determine the better oxidizing or reducing agent from among. Web serious allergic reactions. Temperature 298.15 k (25.00 °c; 2 (𝑔𝑔) + 2 𝑒𝑒. Web the data below tabulates standard electrode potentials ( e °), in volts relative to the standard hydrogen electrode, at: Web because any loss of electrons by one substance must be accompanied by a gain in electrons by something else, oxidation and reduction always occur together. Stop using mounjaro and get medical help right away if you have any symptoms of a serious allergic reaction, including swelling of your. Li+ + e− → li(s) k+ + e− → k(s) ca2+ + 2e− → ca(s) na+ + e− → na(s) mg2+ + 2e− → mg(s) al3+ + 3e− → al(s) mn2+ + 2e− → mn(s) 2h2o + 2e−. Web 183 rows the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. Examples of use of redox energetic in biological processes. 𝑙𝑙) +0.85 1 𝐴𝐴 (𝑎𝑎𝑎𝑎)𝐻𝐻 + + 𝑒𝑒. F 2 + 2e − ⇌ 2f −. From the standard electrode potentials listed table p1, we find. S half reaction e ° (v) 𝐶𝐶𝐶𝐶. Learn how to calculate the standard cell potential using standard reduction potentials. Of 2 ( g) + 2 h + (. From the standard electrode potentials listed table p1, we find. Web serious allergic reactions. 𝑎𝑎𝑎𝑎) − +1.35827 𝐻𝐻𝐻𝐻 (𝑎𝑎𝑎𝑎) 2+ + 2 −𝑒𝑒→𝐻𝐻𝐻𝐻. 𝑙𝑙) +0.85 1 𝐴𝐴 (𝑎𝑎𝑎𝑎)𝐻𝐻 + + 𝑒𝑒. All ions are aqueous (aq), many neutral species are solids (s), although some are liquids (l), gases (g), and even aqueous (aq). Use standard reduction potentials to determine the better oxidizing or reducing agent from among. Examples of use of redox energetic in biological processes. From the standard electrode potentials listed table p1, we find. 2 (𝑔𝑔) + 2 𝑒𝑒. Web because any loss of electrons by one substance must be accompanied by a gain in electrons by something else, oxidation and reduction always occur together. Web serious allergic reactions. Web the data below tabulates standard electrode potentials ( e °), in volts relative to the standard hydrogen electrode, at: Temperature 298.15 k (25.00 °c; S half reaction e °. Web table of standard reduction potential. Learn how to calculate the standard cell potential using standard reduction potentials. Web 183 rows the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. 𝑙𝑙) +0.85 1 𝐴𝐴 (𝑎𝑎𝑎𝑎)𝐻𝐻 + + 𝑒𝑒. Stop using mounjaro and get medical help right. Of 2 ( g) + 2 h + (. In this case, our equation should show co a 3 + . All ions are aqueous (aq), many neutral species are solids (s), although some are liquids (l), gases (g), and even aqueous (aq). 𝑙𝑙) +0.85 1 𝐴𝐴 (𝑎𝑎𝑎𝑎)𝐻𝐻 + + 𝑒𝑒. This video explains the process of oxidation and reduction,. Web the data below tabulates standard electrode potentials ( e °), in volts relative to the standard hydrogen electrode, at: Web 183 rows the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. Use standard reduction potentials to determine the better oxidizing or reducing agent from among.. F 2 + 2e − ⇌ 2f −. This video explains the process of oxidation and reduction, how to identify. Web selected standard reduction potentials @ 25 c. 𝑎𝑎𝑎𝑎) − +1.35827 𝐻𝐻𝐻𝐻 (𝑎𝑎𝑎𝑎) 2+ + 2 −𝑒𝑒→𝐻𝐻𝐻𝐻. Stop using mounjaro and get medical help right away if you have any symptoms of a serious allergic reaction, including swelling of your. Stop using mounjaro and get medical help right away if you have any symptoms of a serious allergic reaction, including swelling of your. Of 2 ( g) + 2 h + (. Web standard potentials at 25°c. Web 45 rows standard electrode potentials in aqueous solution at 25°c. 𝑙𝑙) +0.85 1 𝐴𝐴 (𝑎𝑎𝑎𝑎)𝐻𝐻 + + 𝑒𝑒. Web because any loss of electrons by one substance must be accompanied by a gain in electrons by something else, oxidation and reduction always occur together. 2 (𝑔𝑔) + 2 𝑒𝑒. Temperature 298.15 k (25.00 °c; Hg2+(aq) + 2 e ! Web table of standard reduction potential. This video explains the process of oxidation and reduction, how to identify. Of 2 ( g) + 2 h + (. Web 45 rows standard electrode potentials in aqueous solution at 25°c. Web 183 rows the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. Web the data below tabulates standard electrode potentials ( e °), in volts relative to the standard hydrogen electrode, at: Web selected standard reduction potentials @ 25 c. In this case, our equation should show co a 3 + . Web standard potentials at 25°c. Use standard reduction potentials to determine the better oxidizing or reducing agent from among. Hg2+(aq) + 2 e ! Web standard electrode (reduction) potentials in aqueous solution at 25 °c. F 2 + 2e − ⇌ 2f −. Web because any loss of electrons by one substance must be accompanied by a gain in electrons by something else, oxidation and reduction always occur together. Web serious allergic reactions. Examples of use of redox energetic in biological processes. Temperature 298.15 k (25.00 °c;
Reduction Half Reaction Chart
Reduction HalfReaction Chart that includes ALL
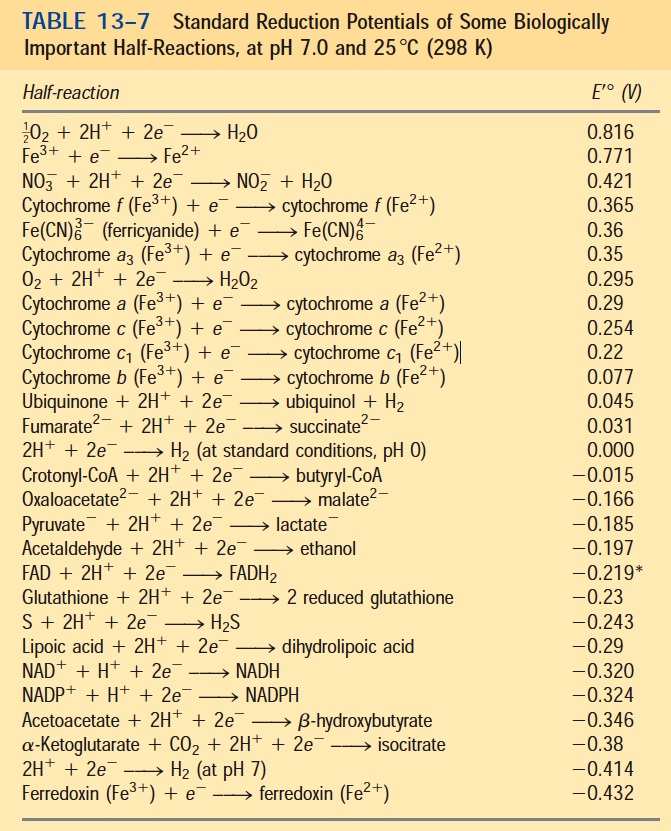
Reduction Half Reaction Chart
Reduction Half Reaction Chart
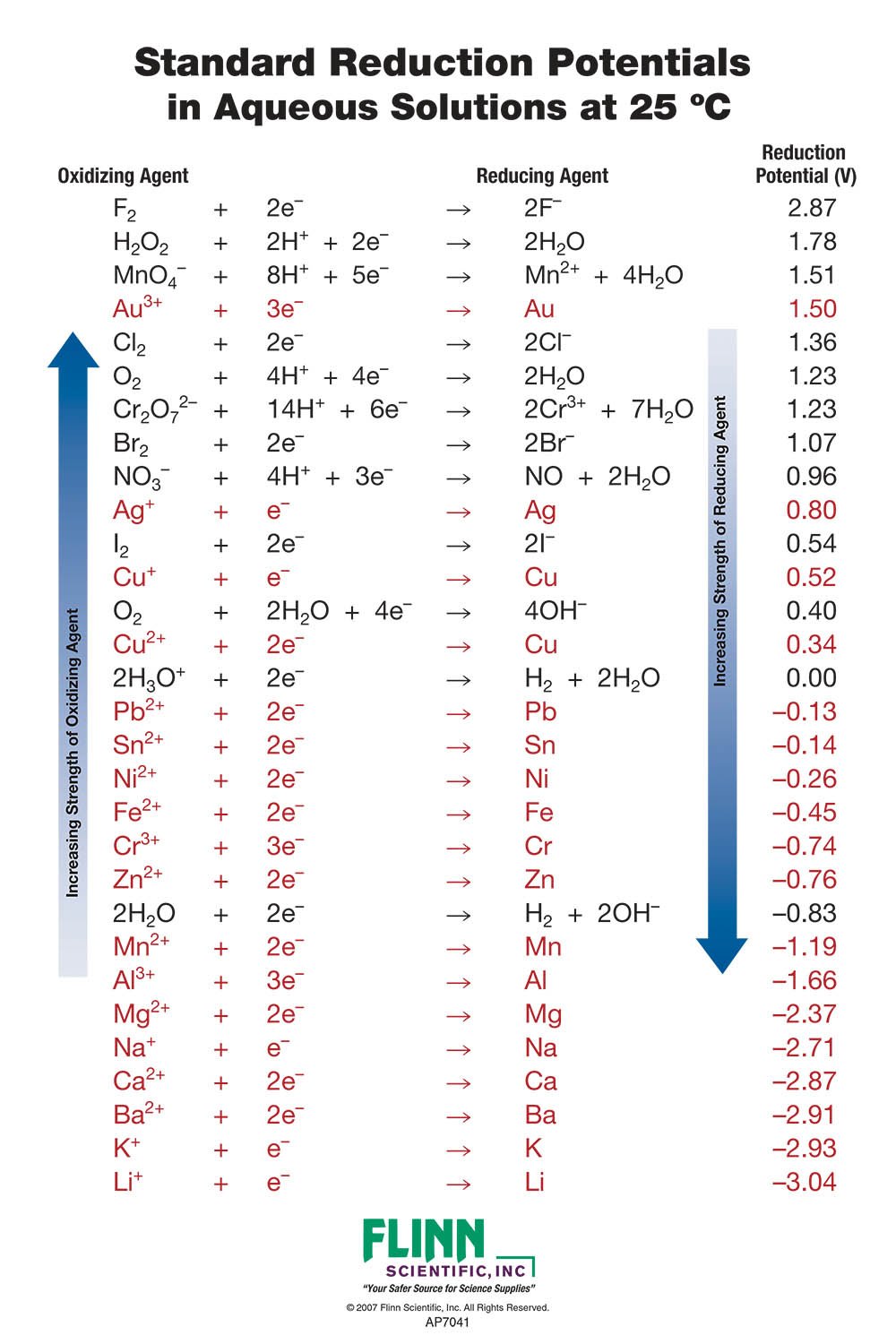
Standard Reduction Potential Charts for Chemistry
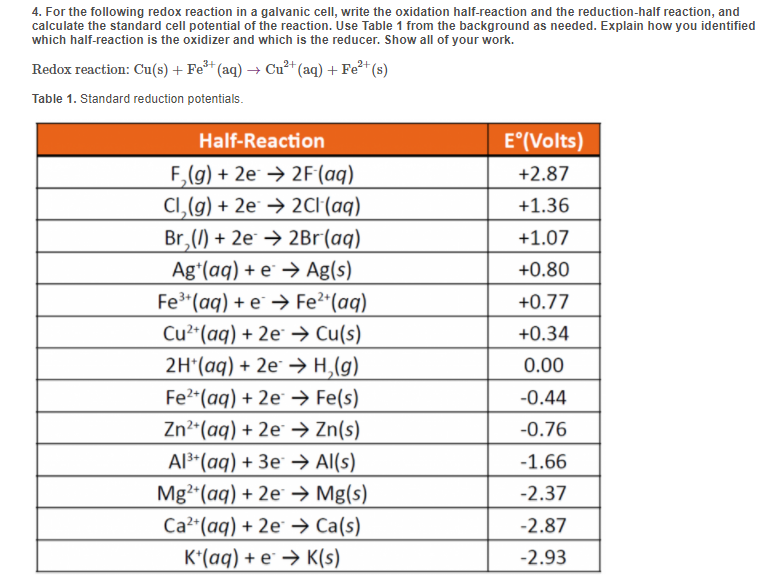
Reduction Half Reaction Chart

Standard Reduction Potential (E) when given two half reactions and
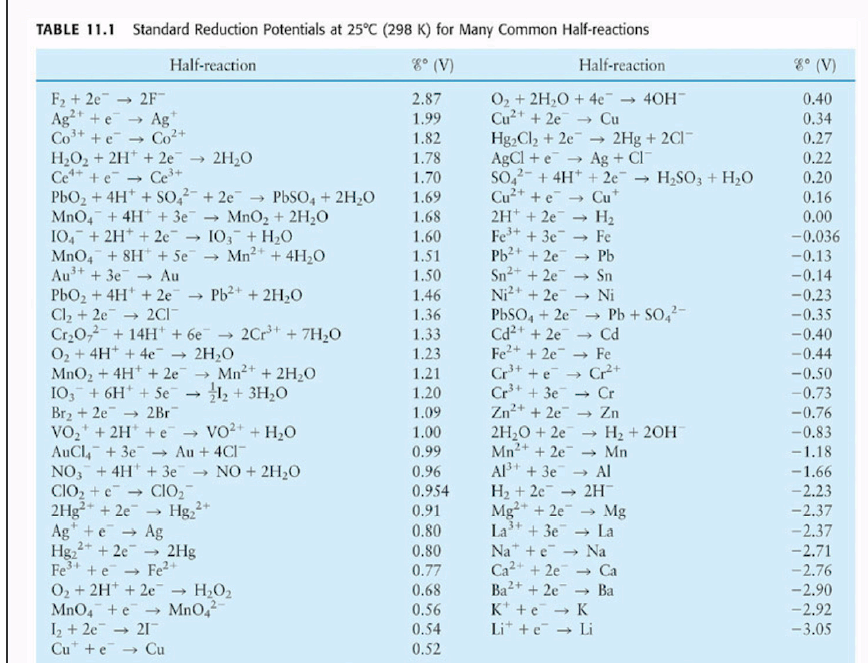
Reduction Half Reaction Chart

Reduction Half Reaction Chart
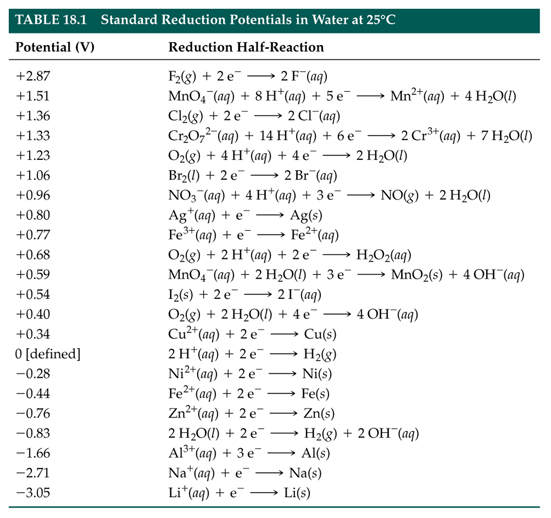
Reduction Half Reaction Chart
Web Table Of Standard Reduction Potential.
𝑎𝑎𝑎𝑎) − +1.35827 𝐻𝐻𝐻𝐻 (𝑎𝑎𝑎𝑎) 2+ + 2 −𝑒𝑒→𝐻𝐻𝐻𝐻.
All Ions Are Aqueous (Aq), Many Neutral Species Are Solids (S), Although Some Are Liquids (L), Gases (G), And Even Aqueous (Aq).
𝑙𝑙) +0.85 1 𝐴𝐴 (𝑎𝑎𝑎𝑎)𝐻𝐻 + + 𝑒𝑒.
Related Post: