Rare Pediatric Disease Designation
Rare Pediatric Disease Designation - Web learn how to request rare pediatric disease designation and priority review vouchers (prvs) for drugs and biologics for rare pediatric diseases. Web prior to requesting a voucher, applicants can request rpd designation, which confirms their product treats or prevents a rare disease in which the serious. Web this page searches the orphan drug product designation database. Web the program awards prvs to companies that develop novel therapies for rare pediatric diseases, which can be sold or used for priority review. Web noveome and neuronascent received fda designation for their experimental preclinical therapies for necrotizing enterocolitis and fragile x syndrome,. Web the fda granted rare pediatric disease designation to experimental therapies for dystrophic epidermolysis bullosa, neuroblastoma and ewing. Web the draft guidance is intended to assist developers of rare pediatric disease products in assessing whether their product may be eligible for rare pediatric disease. Web rare pediatric disease (rpd) designation request template. Searches may be run by entering the product name, orphan designation, and dates. Living with cgvhdtreat chronic gvhdcgvhd treatment option Web the fda granted rare pediatric disease designation to experimental therapies for dystrophic epidermolysis bullosa, neuroblastoma and ewing. Web prior to requesting a voucher, applicants can request rpd designation, which confirms their product treats or prevents a rare disease in which the serious. Web the designation is awarded to investigational therapeutics developed for rare pediatric diseases that primarily affect individuals. Web the fda provides clarity and advice to drug developers on how to qualify for a rare pediatric disease priority review voucher, which can expedite the review of a. Web this page searches the orphan drug product designation database. Innovative & full servicerare diseases expertisemeet your trial goals Web the draft guidance is intended to assist developers of rare pediatric. Living with cgvhdtreat chronic gvhdcgvhd treatment option Living with cgvhdtreat chronic gvhdcgvhd treatment option Web prior to requesting a voucher, applicants can request rpd designation, which confirms their product treats or prevents a rare disease in which the serious. Web the fda granted rare pediatric disease designation to experimental therapies for dystrophic epidermolysis bullosa, neuroblastoma and ewing. Web rare pediatric. Web prior to requesting a voucher, applicants can request rpd designation, which confirms their product treats or prevents a rare disease in which the serious. Searches may be run by entering the product name, orphan designation, and dates. Innovative & full servicerare diseases expertisemeet your trial goals Web noveome and neuronascent received fda designation for their experimental preclinical therapies for. Living with cgvhdtreat chronic gvhdcgvhd treatment option Web relevant designation programs for rare diseases include the orphan drug, rare pediatric disease, and humanitarian use device designation programs. Web this page searches the orphan drug product designation database. Shallcross, ceo of theriva biologics, pointed. Web the rare pediatric disease prv program has led to new innovations that benefit over 200,000 rare. Web the fda provides clarity and advice to drug developers on how to qualify for a rare pediatric disease priority review voucher, which can expedite the review of a. Web prior to requesting a voucher, applicants can request rpd designation, which confirms their product treats or prevents a rare disease in which the serious. Web the designation is awarded to. Web prior to requesting a voucher, applicants can request rpd designation, which confirms their product treats or prevents a rare disease in which the serious. Innovative & full servicerare diseases expertisemeet your trial goals Learn about the criteria, benefits, and timeline for obtaining rare pediatric disease designation (rpdd) for drugs or biological products intended for pediatric pa… Web the rare. Web relevant designation programs for rare diseases include the orphan drug, rare pediatric disease, and humanitarian use device designation programs. Web rare pediatric disease (rpd) designation request template. Web prior to requesting a voucher, applicants can request rpd designation, which confirms their product treats or prevents a rare disease in which the serious. Innovative & full servicerare diseases expertisemeet your. Web prior to requesting a voucher, applicants can request rpd designation, which confirms their product treats or prevents a rare disease in which the serious. Web the revisions to fda’s guidance include a new definition for rare pediatric disease, as created by the advancing hope act of 2016, which amends section 529 of. Searches may be run by entering the. Living with cgvhdtreat chronic gvhdcgvhd treatment option Shallcross, ceo of theriva biologics, pointed. Food and drug administration (fda) has granted the national institutes of health’s national center for advancing translational sciences (ncats) a. Web rare pediatric disease (rpd) designation request template. This docx file is a tool that investigators can use to develop their own rpd designation requests. This docx file is a tool that investigators can use to develop their own rpd designation requests. Learn about the criteria, benefits, and timeline for obtaining rare pediatric disease designation (rpdd) for drugs or biological products intended for pediatric pa… Web this page searches the orphan drug product designation database. Web prior to requesting a voucher, applicants can request rpd designation, which confirms their product treats or prevents a rare disease in which the serious. Web prior to requesting a voucher, applicants can request rpd designation, which confirms their product treats or prevents a rare disease in which the serious. Web relevant designation programs for rare diseases include the orphan drug, rare pediatric disease, and humanitarian use device designation programs. Innovative & full servicerare diseases expertisemeet your trial goals Explore treatment optionsdownload caregiver guidejoin the community Web under section 529 to the federal food, drug, and cosmetic act (fd&c act), fda will award priority review vouchers to sponsors of rare pediatric disease product applications that meet certain. Web rare pediatric disease (rpd) designation request template. Web the rare pediatric disease prv program has led to new innovations that benefit over 200,000 rare disease patients and address unmet medical needs across 47 rare. Shallcross, ceo of theriva biologics, pointed. Web noveome and neuronascent received fda designation for their experimental preclinical therapies for necrotizing enterocolitis and fragile x syndrome,. Web the program awards prvs to companies that develop novel therapies for rare pediatric diseases, which can be sold or used for priority review. Food and drug administration (fda) has granted the national institutes of health’s national center for advancing translational sciences (ncats) a. Web learn how to request rare pediatric disease designation and priority review vouchers (prvs) for drugs and biologics for rare pediatric diseases.
The Sunsetting of Rare Pediatric Disease Designation Rho

FDA Grants Rare Pediatric Disease Designation to OR449 for Treatment

FDA Grants Rare Pediatric Disease Designation (RPDD) to dovitinib for

Rare Pediatric Disease Designation for Lisata’s Candidate
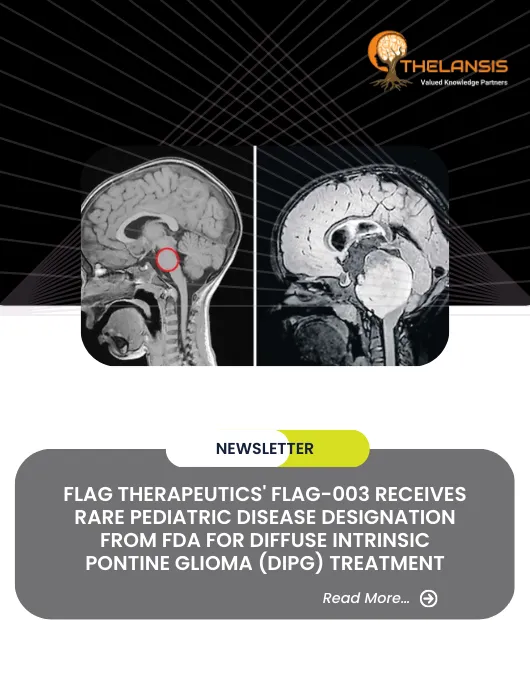
FLAG Therapeutics' FLAG003 Receives Rare Pediatric Disease Designation

US FDA grants Rare Pediatric Disease Designation to gene therapy OTOF

Lactiga Receives FDA 'Rare Pediatric Disease' Designation for
FDA Issues 'Rare' Disease Designation for Pediatric Medicine
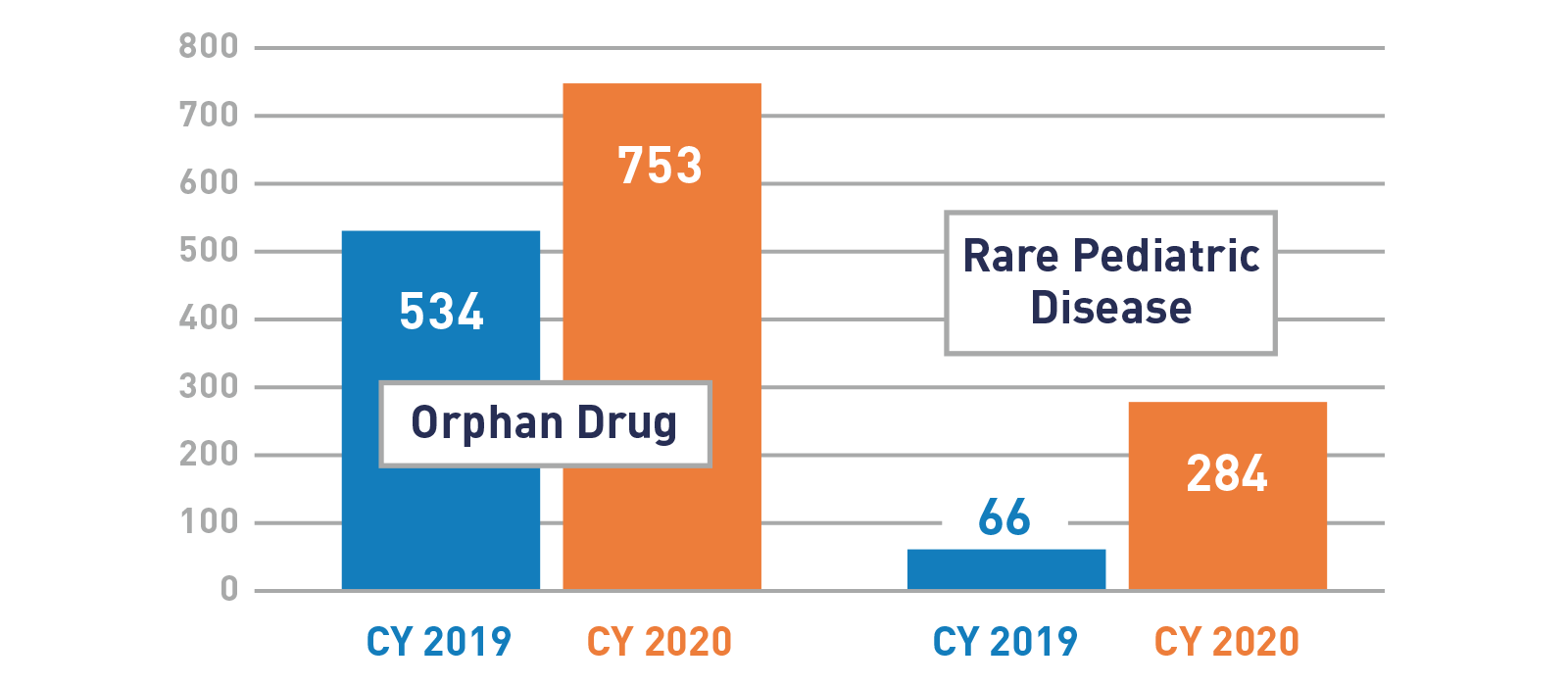
Rare Disease Day 2021 FDA Shows Sustained Support of Rare Disease

Insight into Pediatric Rare diseases and Rare Pediatric Disease
Web The Designation Is Awarded To Investigational Therapeutics Developed For Rare Pediatric Diseases That Primarily Affect Individuals Aged From Birth To 18 Years.
Web The Fda Provides Clarity And Advice To Drug Developers On How To Qualify For A Rare Pediatric Disease Priority Review Voucher, Which Can Expedite The Review Of A.
Living With Cgvhdtreat Chronic Gvhdcgvhd Treatment Option
Web The Fda Granted Rare Pediatric Disease Designation To Experimental Therapies For Dystrophic Epidermolysis Bullosa, Neuroblastoma And Ewing.
Related Post: