Precedex Dosing Chart
Precedex Dosing Chart - For adult patients, a maintenance infusion of 0.2 to 0.7 mcg/kg/hour is recommended. Web dexmedetomidine is considered safe for sedation during certain procedures. It is to be used only when conventional sedation (propofol, remifentanyl, clonidine) fail to adequately manage patients to the desired sedation (rass) score or in patients with agitation or delirium where weaning o. • precedex should be administered. Plus renal, liver and dialysis adjustments. Web • precedex dosing should be individualized and titrated to desired clinical response. • precedex is not indicated for infusions lasting longer than 24 hours. Each ml contains 118 mcg of dexmedetomidine hydrochloride (equivalent to 100 mcg or 0.1 mg of dexmedetomidine). Web • precedex dosing should be individualized and titrated to desired clinical response. Web as a result, selection of an agent must be individualized according to pharmacokinetic variables (eg, potential interactions with other drugs) and nonpharmacokinetic variables (eg, kidney and liver failure, presence of bradycardia or hypotension), the etiology of the distress, and the desired depth of sedation. • precedex should be administered. Dexmedetomidine hydrochloride safely and effectively. Web • precedex dosing should be individualized and titrated to desired clinical response. • precedex is not indicated for infusions lasting longer than 24 hours. • precedex is not indicated for infusions lasting longer than 24 hours. Web • precedex dosing should be individualized and titrated to desired clinical response. Includes dose adjustments, warnings and precautions. • precedex is not indicated for infusions lasting longer than 24 hours. Last updated on may 16, 2024. The precedex® infusion should not generally exceed 24 hours. Additional case information was obtained through chart review. Im to extubate has proven d. Web • precedex is indicated for sedation of initially intubated and mechanically ventilated patients during treatment in an intensive care setting and for sedation of nonintubated patients prior to and/or during surgical and other procedures.1 • precedex should be administered by continuous infusion not to exceed. Dexmedetomidine hydrochloride safely and effectively. Web when used in anesthesia, the typical dosing is a loading dose of 0.5 to 1.0 mcg/kg, usually followed by a continuous infusion of 0.2 to 0.7 mcg/kg per hour titrated to desired sedation goals. Web • precedex is indicated for sedation of initially intubated and mechanically ventilated patients during treatment in an intensive care. Package insert / product label. For adult patients, a maintenance infusion of 0.2 to 0.7 mcg/kg/hour is recommended. • precedex is not indicated for infusions lasting longer than 24 hours. Web detailed dosage guidelines and administration information for precedex (dexmedetomidine hydrochloride). Web as a result, selection of an agent must be individualized according to pharmacokinetic variables (eg, potential interactions with. • precedex is not indicated for infusions lasting longer than 24 hours. The rate of the maintenance infusion should be adjusted to achieve the desired level of sedation for optimal clinical effect. Web as a result, selection of an agent must be individualized according to pharmacokinetic variables (eg, potential interactions with other drugs) and nonpharmacokinetic variables (eg, kidney and liver. (median total dose = 2 mg), one of whom received an infusion. • precedex is not indicated for infusions lasting longer than 24 hours. Web detailed dexmedetomidine dosage information for adults and the elderly. Package insert / product label. Web precedex® is indicated for sedation of initially intubated and mechanically ventilated patients during treatment in an intensive care setting by. Web precedex® is indicated for sedation of initially intubated and mechanically ventilated patients during treatment in an intensive care setting by continuous intravenous infusion. Additional case information was obtained through chart review. Web • precedex dosing should be individualized and titrated to desired clinical response. The rate of the maintenance infusion should be adjusted to achieve the desired level of. Web • precedex dosing should be individualized and titrated to desired clinical response. Includes dose adjustments, warnings and precautions. • precedex is not indicated for infusions lasting longer than 24 hours. Each ml contains 118 mcg of dexmedetomidine hydrochloride (equivalent to 100 mcg or 0.1 mg of dexmedetomidine). A single in dose of 1 µg/kg is most frequently used. Web detailed dosage guidelines and administration information for precedex (dexmedetomidine hydrochloride). For adult patients, it is recommended that administration of dexmedetomidine hydrochloride starts with a 1.0 mcg/kg loading dose administered over 10 minutes followed by a maintenance dose. Includes dose adjustments, warnings and precautions. Web precedex® is indicated for sedation of initially intubated and mechanically ventilated patients during treatment in. Dexmedetomidine hydrochloride safely and effectively. (median total dose = 2 mg), one of whom received an infusion. • precedex is not indicated for infusions lasting longer than 24 hours. Web detailed dexmedetomidine dosage information for adults and the elderly. Web dexmedetomidine hydrochloride (monograph) brand names: For awake fiberoptic intubation in adult patients: For adult patients, it is recommended that administration of dexmedetomidine hydrochloride starts with a 1.0 mcg/kg loading dose administered over 10 minutes followed by a maintenance dose. The precedex® infusion should not generally exceed 24 hours. Last updated on may 16, 2024. All patients’ neurologic exams were normal by 4 hours after arrival in an ed. Each ml contains 118 mcg of dexmedetomidine hydrochloride (equivalent to 100 mcg or 0.1 mg of dexmedetomidine). Package insert / product label. • precedex should be administered. Im to extubate has proven d. Plus renal, liver and dialysis adjustments. Enoceptor agonist with sedative and analgesic properties.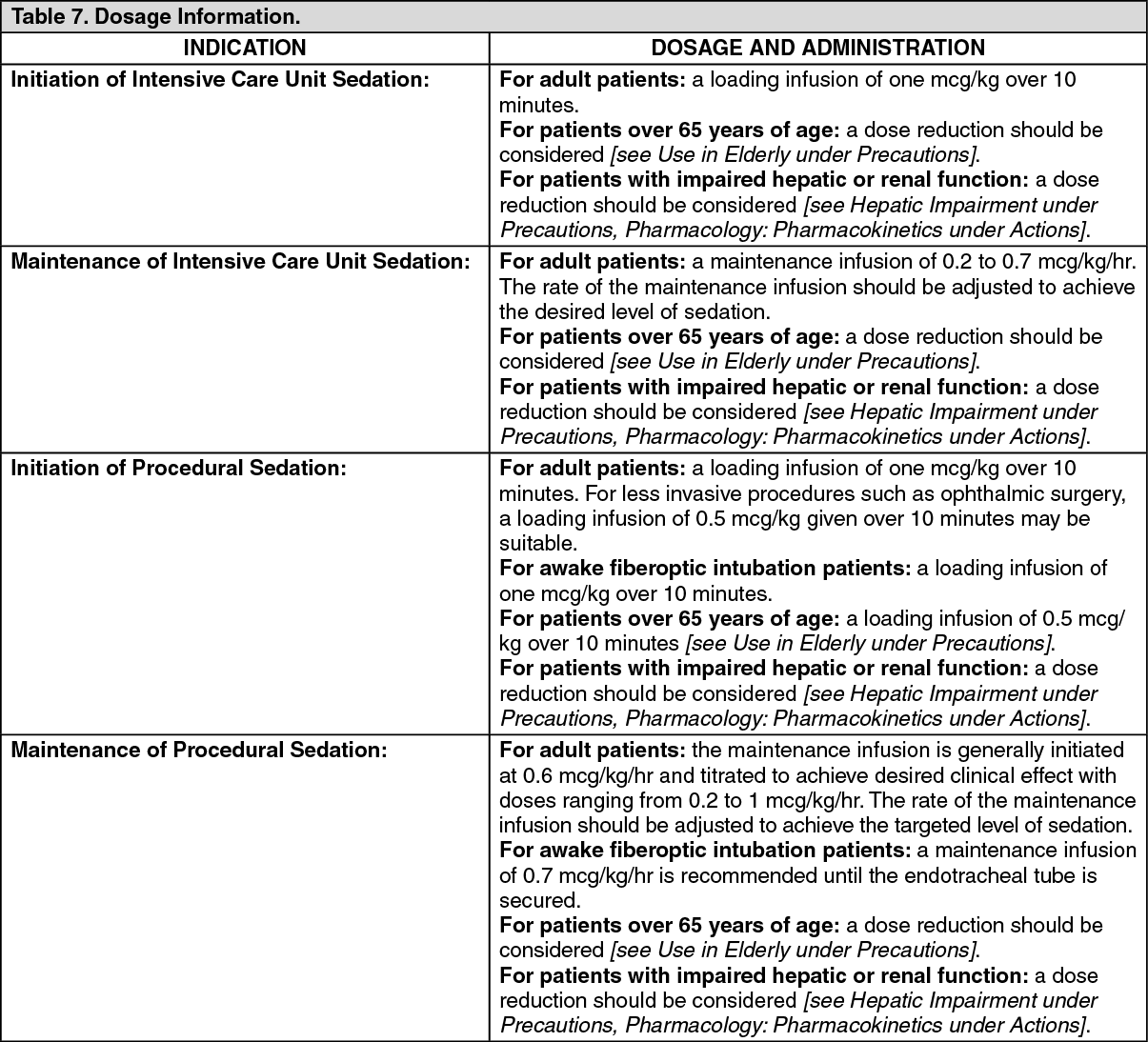
Precedex Full Prescribing Information, Dosage & Side Effects MIMS

Precedex Package Insert

NDC 04091660 Precedex Dexmedetomidine Hydrochloride Injection

Precedex Dosing Basics, How to Dose Precedex Precedex
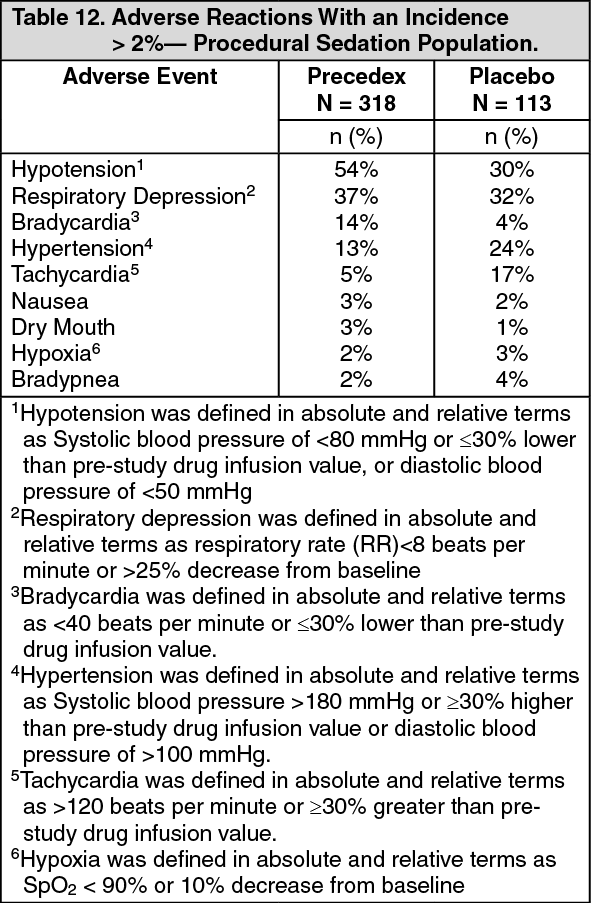
Precedex Full Prescribing Information, Dosage & Side Effects MIMS
Precedex FDA prescribing information, side effects and uses
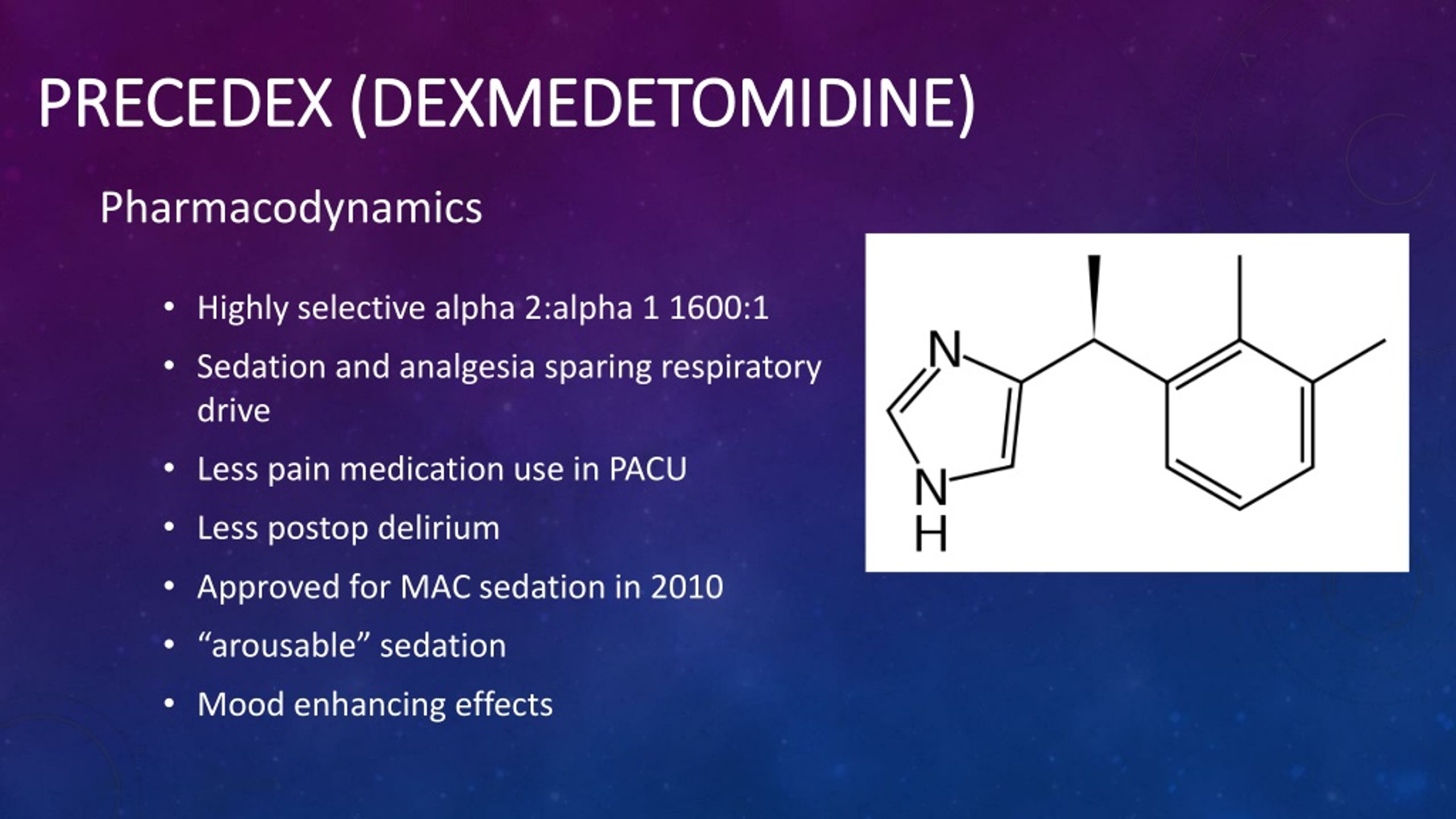
Dexmedetomidine Dosing Chart
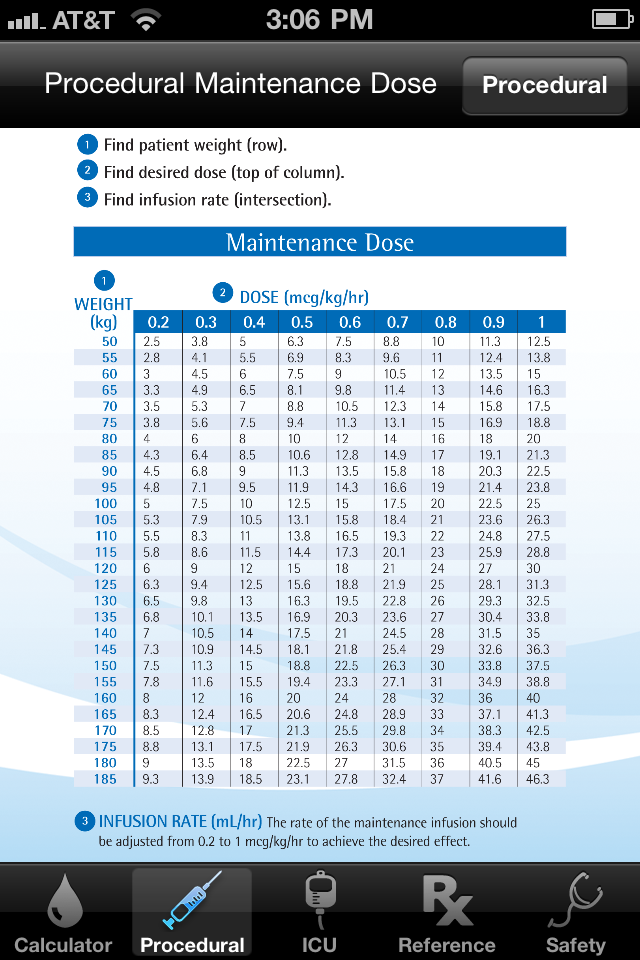
App Shopper Precedex Dexmedetomidine (Medical)

NDC 52584638 Precedex Dexmedetomidine Hydrochloride
Precedex Dosing PDF Intravenous Therapy Dose (Biochemistry)
A Loading Infusion Of 1.0 Mcg/Kg Over 10 Minutes May Be Suitable.
Web Dosing Guidelines • Precedex Dosing Should Be Individualized And Titrated To Desired Clinical Response.
Web Detailed Dosage Guidelines And Administration Information For Precedex Concentrate (Dexmedetomidine Hydrochloride).
Web • Precedex Dosing Should Be Individualized And Titrated To Desired Clinical Response.
Related Post:
