Post Market Surveillance Plan Template
Post Market Surveillance Plan Template - Why is postmarket surveillance so important? How does postmarket surveillance fit. If you are a user of formwork, our eqms software, you. This is a free template, provided by openregulatory. Web a post market surveillance plan (pms plan) is a systematic plan of the processes and the activities to continuously monitor the safety and the performance of the medical devices. The template outlines the content, process and. The medical devices regulation (mdr) (regulation (eu) 2017/745) and in vitro medical device regulation (ivdr). Web the pms template provides a structured approach to setting up a pms process, defining the responsibilities of each team member, and outlining how feedback will be gathered. The document is fully editable so that you can adapt it to your company design. Web table of contents. Web the pms template provides a structured approach to setting up a pms process, defining the responsibilities of each team member, and outlining how feedback will be gathered. The template outlines the content, process and. If you are a user of formwork, our eqms software, you. The new template is now integrated with risk management requirements to include the risk.. How does postmarket surveillance fit. This is a free template, provided by openregulatory. Web section 522 of the federal food, drug, and cosmetic act (fd&c act) provides the food and drug administration (fda) with the authority to require manufacturers to conduct. Why is postmarket surveillance so important? The template outlines the content, process and. What is postmarket surveillance of medical devices? Web a post market surveillance plan (pms plan) is a systematic plan of the processes and the activities to continuously monitor the safety and the performance of the medical devices. How to avoid the 7 most common mistakes. Web table of contents. The document is fully editable so that you can adapt it. Why is postmarket surveillance so important? The template outlines the content, process and. This is a free template, provided by openregulatory. Web section 522 of the federal food, drug, and cosmetic act (fd&c act) provides the food and drug administration (fda) with the authority to require manufacturers to conduct. If you are a user of formwork, our eqms software, you. What is postmarket surveillance of medical devices? If you are a user of formwork, our eqms software, you. Web section 522 of the federal food, drug, and cosmetic act (fd&c act) provides the food and drug administration (fda) with the authority to require manufacturers to conduct. Web a post market surveillance plan (pms plan) is a systematic plan of the. Web a post market surveillance plan (pms plan) is a systematic plan of the processes and the activities to continuously monitor the safety and the performance of the medical devices. Why is postmarket surveillance so important? The medical devices regulation (mdr) (regulation (eu) 2017/745) and in vitro medical device regulation (ivdr). If you are a user of formwork, our eqms. If you are a user of formwork, our eqms software, you. This is a free template, provided by openregulatory. The new template is now integrated with risk management requirements to include the risk. The medical devices regulation (mdr) (regulation (eu) 2017/745) and in vitro medical device regulation (ivdr). Documents include placeholder marks for all. This is a free template, provided by openregulatory. Documents include placeholder marks for all. The template outlines the content, process and. How does postmarket surveillance fit. The new template is now integrated with risk management requirements to include the risk. Documents include placeholder marks for all. How does postmarket surveillance fit. Web section 522 of the federal food, drug, and cosmetic act (fd&c act) provides the food and drug administration (fda) with the authority to require manufacturers to conduct. Web the pms template provides a structured approach to setting up a pms process, defining the responsibilities of each team member,. Web a post market surveillance plan (pms plan) is a systematic plan of the processes and the activities to continuously monitor the safety and the performance of the medical devices. How does postmarket surveillance fit. The template outlines the content, process and. The document is fully editable so that you can adapt it to your company design. Web the pms. Web section 522 of the federal food, drug, and cosmetic act (fd&c act) provides the food and drug administration (fda) with the authority to require manufacturers to conduct. How to avoid the 7 most common mistakes. Why is postmarket surveillance so important? The new template is now integrated with risk management requirements to include the risk. What is postmarket surveillance of medical devices? Web the pms template provides a structured approach to setting up a pms process, defining the responsibilities of each team member, and outlining how feedback will be gathered. The template outlines the content, process and. Documents include placeholder marks for all. Web table of contents. If you are a user of formwork, our eqms software, you. The document is fully editable so that you can adapt it to your company design. The medical devices regulation (mdr) (regulation (eu) 2017/745) and in vitro medical device regulation (ivdr).
Post Market Surveillance Plan Template

Postmarket surveillance is in itself a monitoring and measuring

Post Market Surveillance Plan Template

Post Market Surveillance Plan Template

Post Market Surveillance Plan PMS Plan Template

Post Market Surveillance Plan Template

ISO 204162020 Post Market Surveillance Medical Device Manufacturers

(PDF) EU postmarket surveillance plans for medical devices
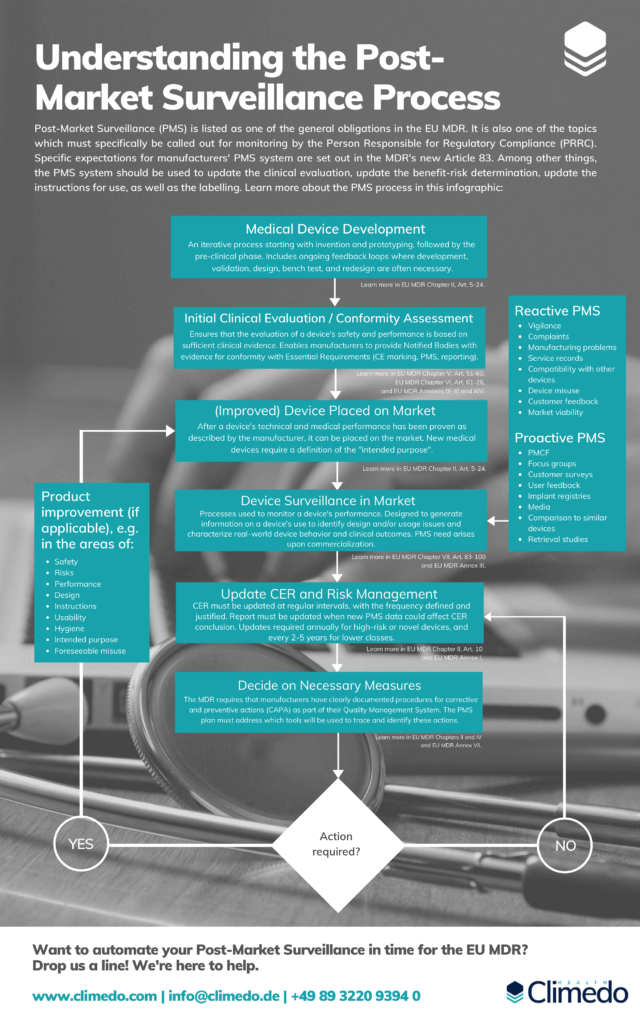
Getting your PostMarket Surveillance up to Speed with the EU MDR
Output of the postmarket surveillance (PMS) plan [Colour figure can be
Web A Post Market Surveillance Plan (Pms Plan) Is A Systematic Plan Of The Processes And The Activities To Continuously Monitor The Safety And The Performance Of The Medical Devices.
This Is A Free Template, Provided By Openregulatory.
How Does Postmarket Surveillance Fit.
Related Post: