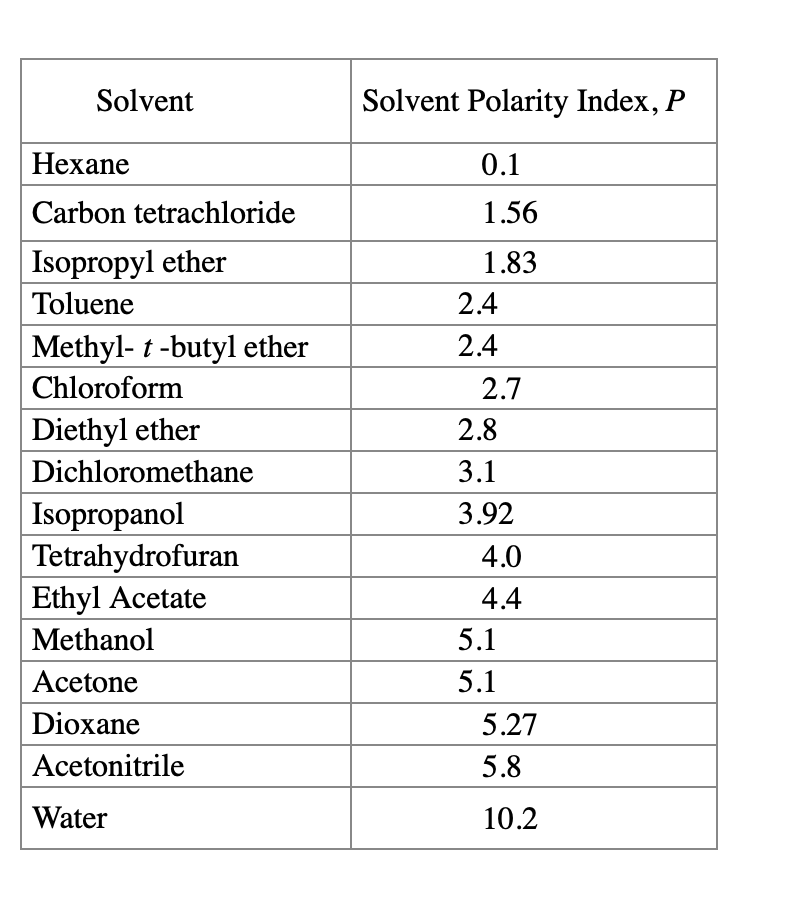
Polarity Of Solvents Chart
Polarity Of Solvents Chart - Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Learn how to use these parameters to select solvents for chromatography and other applications. In order to understand why salts dissolve in water, we have to first understand solvent polarity. Web explain the concepts of polar covalent bonds and molecular polarity. Web were the results on solvent miscibility consistent with solvent polarities? 641 shows some of the most common crystallization. Web 24 rows find the polarity indexes of solvents commonly used for size exclusion. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were extracted from christian reichardt,. Laboratory techniques and methods to. Web as the solvents become more polar, the light absorbed by this dye shifts from the low energy, long wavelength (red) to the high energy, short wavelength (violet). Web 24 rows find the polarity indexes of solvents commonly used for size exclusion. Web were the results on solvent miscibility consistent with solvent polarities? Laboratory techniques and methods to. Web 1 the polarity index is a measure of the relative polarity of a solvent and is useful for identifying suitable mobile phase solvents. Web another parameter commonly manipulated in. Web properties of solvents used in organic chemistry including mp, bp, desnity, water solubiity, polarity viscosity, dipole moment, dielectric constant Web 8.3 polarity of solvents. Web 1 the polarity index is a measure of the relative polarity of a solvent and is useful for identifying suitable mobile phase solvents. Web were the results on solvent miscibility consistent with solvent polarities?. Web burdick & jackson solvents are arranged in order of increasing polarity index, a relative measure of the degree of interaction of the solvent with various polar test solutes. Differences in the polarity of solvents. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were extracted from christian reichardt,. Web as the solvents. It also includes charts on relative solvent. Differences in the polarity of solvents. Web properties of solvents used in organic chemistry including mp, bp, desnity, water solubiity, polarity viscosity, dipole moment, dielectric constant Web 24 rows find the polarity indexes of solvents commonly used for size exclusion. Assess the polarity of a molecule based on its bonding and structure. However, novel and new substances or solvent combinations are. Web burdick & jackson solvents are arranged in order of increasing polarity index, a relative measure of the degree of interaction of the solvent with various polar test solutes. The following video explains why water (a solvent) is polar. Web find the polarity index (p1) and solvent group of common liquids. Laboratory techniques and methods to. Web today, we will explore how polar organic solvents are, and will test what mixes with what. Web information on the properties of common solvents used in organic chemistry including boiling points, solubility, density, dielectric constants, and flash points The following video explains why water (a solvent) is polar. Common solvents arranged from the least. Laboratory techniques and methods to. Assess the polarity of a molecule based on its bonding and structure. Web today, we will explore how polar organic solvents are, and will test what mixes with what. 641 shows some of the most common crystallization. The polarity index increases with polarity. In order to understand why salts dissolve in water, we have to first understand solvent polarity. The following video explains why water (a solvent) is polar. Web explain the concepts of polar covalent bonds and molecular polarity. It also includes charts on relative solvent. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Web 23 rows polar protic and aprotic solvents. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were extracted from christian reichardt,. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Differences in the polarity of solvents. Common solvents arranged from the least polar to the most polar This page uses frames, but your browser doesn't support them. In order to understand why salts dissolve in water, we have to first understand solvent polarity. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were extracted from christian reichardt,. Web find the polarity index (p1) and solvent group of common liquids in. In order to understand why salts dissolve in water, we have to first understand solvent polarity. However, novel and new substances or solvent combinations are. Web as the solvents become more polar, the light absorbed by this dye shifts from the low energy, long wavelength (red) to the high energy, short wavelength (violet). The following video explains why water (a solvent) is polar. Web 8.3 polarity of solvents. 641 shows some of the most common crystallization. Web burdick & jackson solvents are arranged in order of increasing polarity index, a relative measure of the degree of interaction of the solvent with various polar test solutes. Web were the results on solvent miscibility consistent with solvent polarities? Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were extracted from christian reichardt,. Web 1 the polarity index is a measure of the relative polarity of a solvent and is useful for identifying suitable mobile phase solvents. Web information on the properties of common solvents used in organic chemistry including boiling points, solubility, density, dielectric constants, and flash points This page uses frames, but your browser doesn't support them. Laboratory techniques and methods to. Web the document lists various solvents and provides their polarity index, viscosity, uv cutoff wavelength, solubility in water, and miscibility. Some compounds are clearly very polar (e.g.
Polarity Chart Of Solvents

Solvent Polarity Chart

Solvent Polarity Chart Minga

Polarity Chart Of Solvents
Solved Determine the solvent polarity index for each HPLC
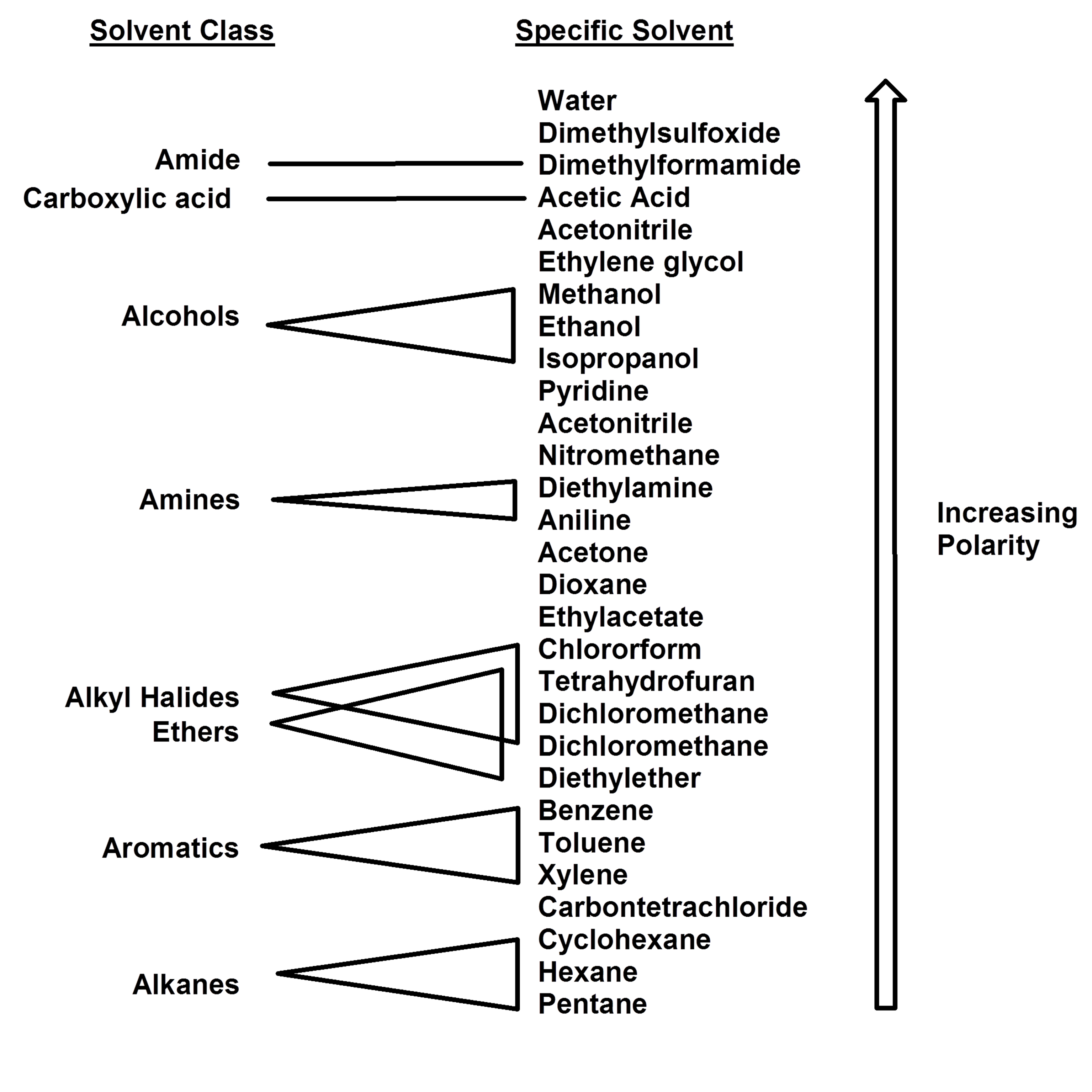
Polarity Of Solvents Chart

Polarity Index Chart
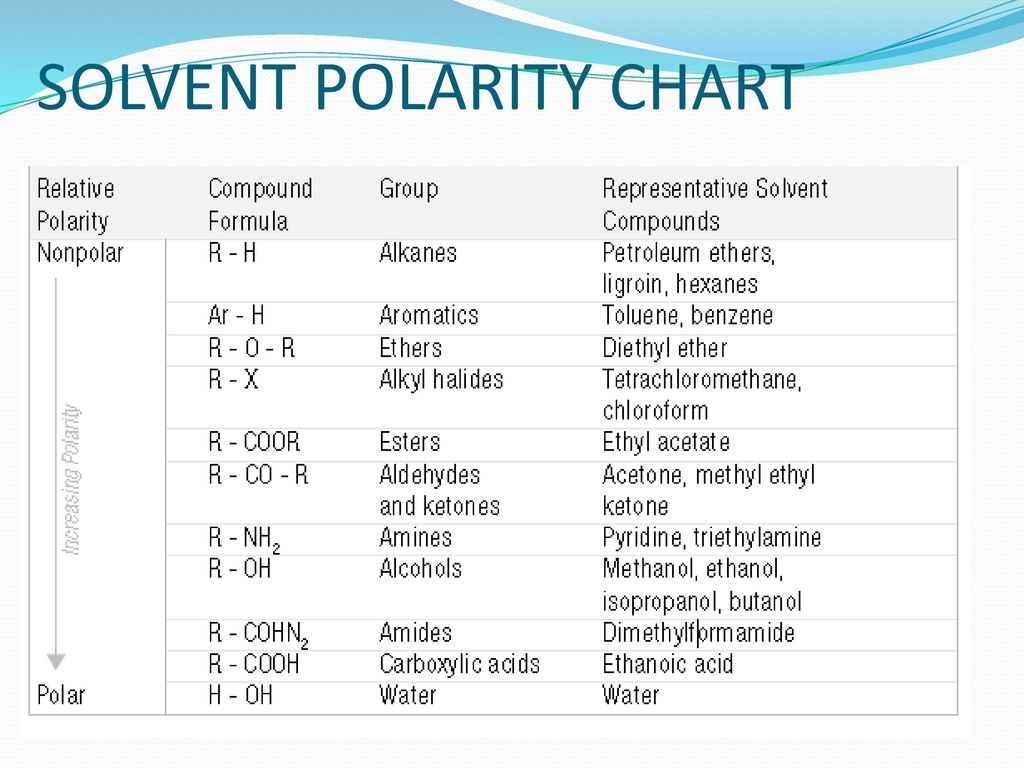
Solvent Polarity Chart For Tlc

Solvent Polarity Chart A Visual Reference of Charts Chart Master
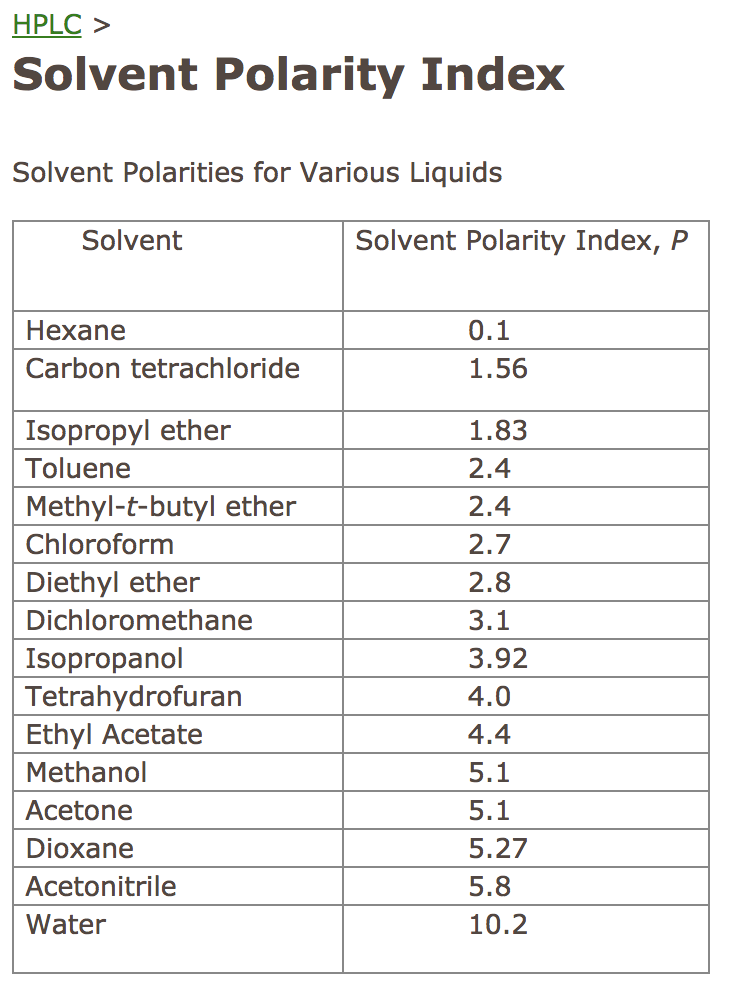
Hplc Solvent Polarity Chart A Visual Reference of Charts Chart Master
Web Find The Polarity Index (P1) And Solvent Group Of Common Liquids In A Table.
Differences In The Polarity Of Solvents.
Web Today, We Will Explore How Polar Organic Solvents Are, And Will Test What Mixes With What.
Web Explain The Concepts Of Polar Covalent Bonds And Molecular Polarity.
Related Post: