Polarity Chart Of Solvents
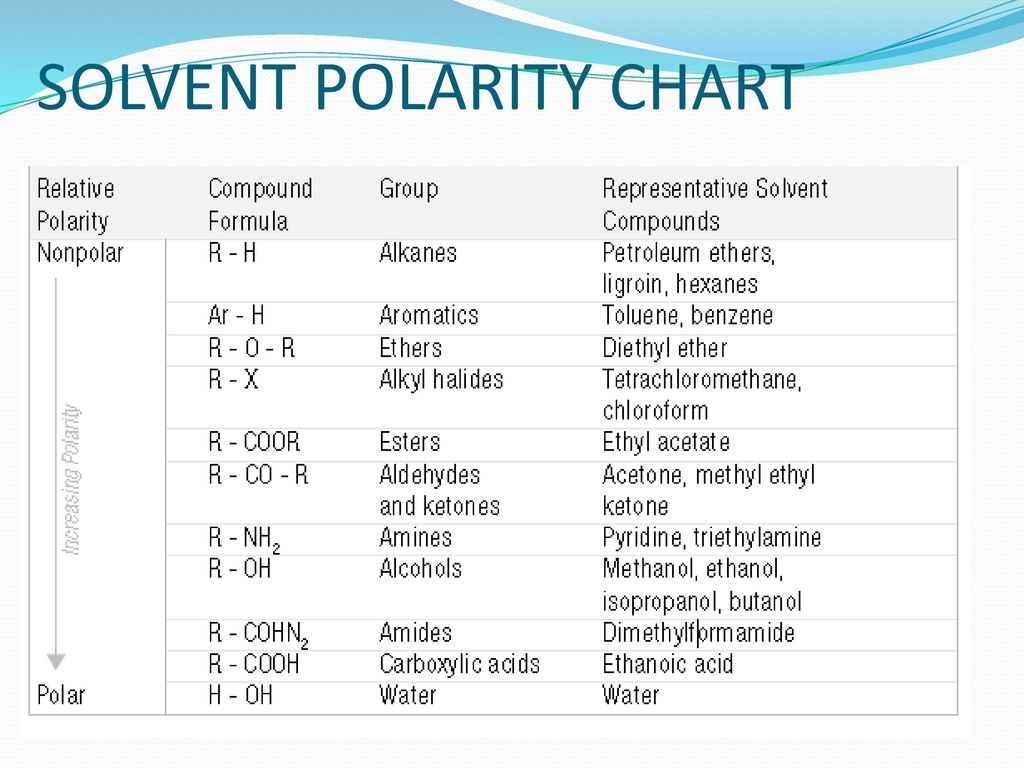
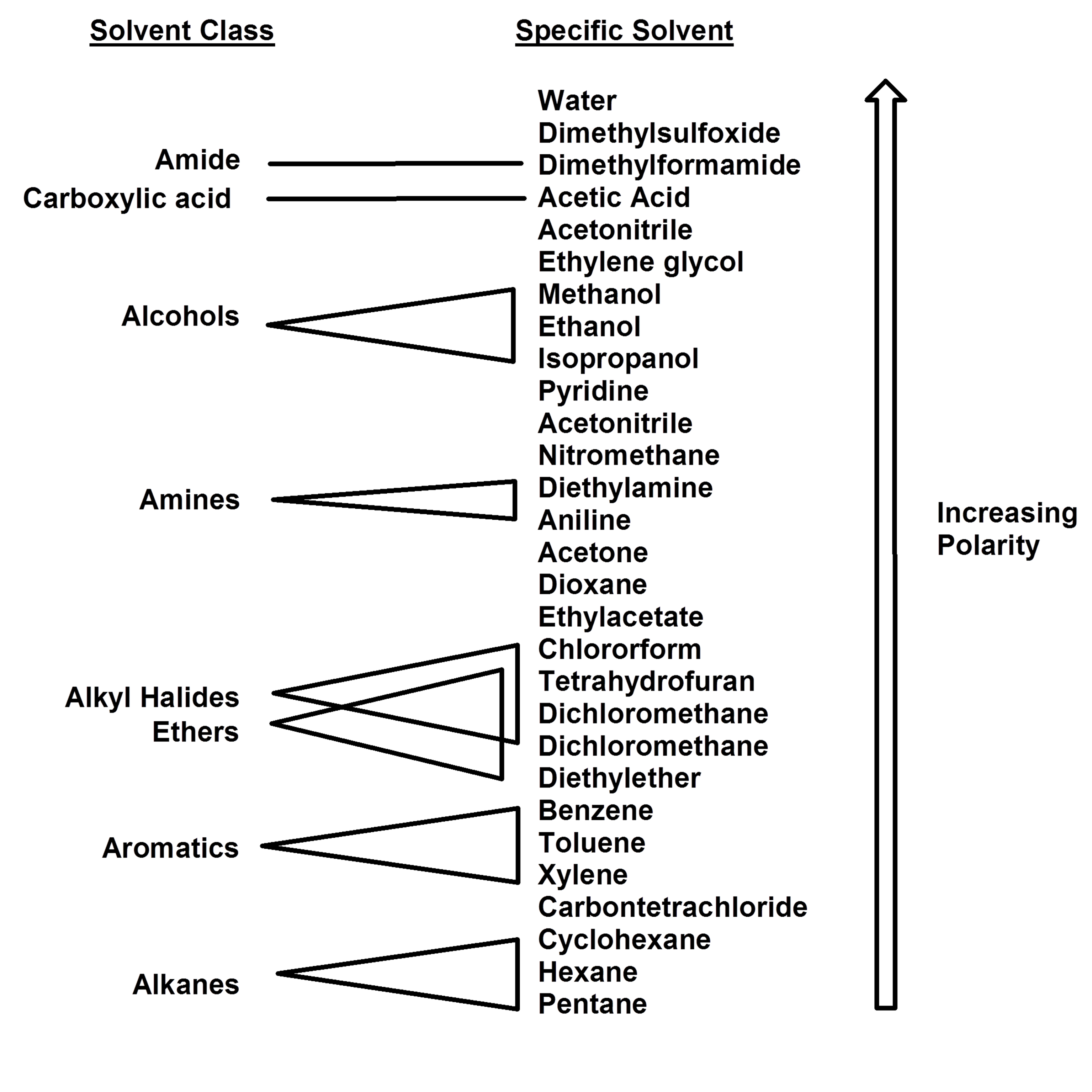
Polarity Chart Of Solvents - Demystifying synthetic organic chemistry since 2004. Water most polar acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane tetrahydrofuran dicholoromethane chloroform diethyl ether benzene toluene xylene. For reverse phase chromatography eluent strength decreases as its polarity increases 2 uv cutoff, the wavelength at which the solvent absorbance in Oil), but many compounds are somewhere in between, and mix with both. References • classification of the solvent properties of common liquids. Web explain the concepts of polar covalent bonds and molecular polarity. Web polar protic and aprotic solvents. Assess the polarity of a molecule based on its bonding and structure. P1 increases with solvent polarity. Web how the concept of solvent polarity investigated with solvatochromic probes helps studying intermolecular interactions. It also includes charts on relative solvent polarity and miscibility/viscosity to aid in selecting suitable mobile phase solvents for chromatography. Web 8.3 polarity of solvents. 641 shows some of the most common crystallization solvents arranged by order of decreasing polarity going from top to bottom. Differences in the polarity of solvents. The following solvents are added to the dye. It also includes charts on relative solvent polarity and miscibility/viscosity to aid in selecting suitable mobile phase solvents for chromatography. Web 8.3 polarity of solvents. Bond dipole moment measurements can be found on individual solvent pages in our solvent center. Web polarity indexes of solvents which are commonly used for sec analysis is shown below. Web explain the concepts of. References • classification of the solvent properties of common liquids. Laboratory techniques and methods to improve your experimental skills. Demystifying synthetic organic chemistry since 2004. Web 8.3 polarity of solvents. Common solvents arranged from the least polar to the most polar Web chemists approximate the vector sum of a molecule's bond dipole moments to be an estimate of the molecule's polarity. Web in this article, we will look at mobile phase solvent preparation, solvent choices, the effect of solvent polarity on hplc separations and solvent purity for various retention modes. Water most polar acetic acid ethylene glycol methanol ethanol isopropanol pyridine. Web another parameter commonly manipulated in crystallization experiments is solvent polarity. Web burdick & jackson solvents are arranged in order of increasing polarity index, a relative measure of the degree of interaction of the solvent with various polar test solutes. Some compounds are clearly very polar (e.g. Web how the concept of solvent polarity investigated with solvatochromic probes helps studying. Bond dipole moment measurements can be found on individual solvent pages in our solvent center. Web in this article, we will look at mobile phase solvent preparation, solvent choices, the effect of solvent polarity on hplc separations and solvent purity for various retention modes. Assess the polarity of a molecule based on its bonding and structure. Web as the solvents. Solvents used in organic chemistry are characterized by their physical characteristics. For reverse phase chromatography eluent strength decreases as its polarity increases 2 uv cutoff, the wavelength at which the solvent absorbance in Common solvents arranged from the least polar to the most polar Water) or quite nonpolar (e.g. Web burdick & jackson solvents are arranged in order of increasing. Common solvents arranged from the least polar to the most polar For reverse phase chromatography eluent strength decreases as its polarity increases 2 uv cutoff, the wavelength at which the solvent absorbance in The following solvents are added to the dye. Web polar protic and aprotic solvents. Web information on the properties of common solvents used in organic chemistry including. Web polarity index (p1) defined as a measure of the ability of the solvent to interact with various test solutes. Web properties of solvents used in organic chemistry including mp, bp, desnity, water solubiity, polarity viscosity, dipole moment, dielectric constant P1 increases with solvent polarity. Web 8.3 polarity of solvents. Web today, we will explore how polar organic solvents are,. Web 1 the polarity index is a measure of the relative polarity of a solvent and is useful for identifying suitable mobile phase solvents. Web polar protic and aprotic solvents. Web as the solvents become more polar, the light absorbed by this dye shifts from the low energy, long wavelength (red) to the high energy, short wavelength (violet) end of. Differences in the polarity of solvents. Web as the solvents become more polar, the light absorbed by this dye shifts from the low energy, long wavelength (red) to the high energy, short wavelength (violet) end of the spectrum. The following solvents are added to the dye. Web chemists approximate the vector sum of a molecule's bond dipole moments to be an estimate of the molecule's polarity. Bond dipole moment measurements can be found on individual solvent pages in our solvent center. Solvents used in organic chemistry are characterized by their physical characteristics. Web 8.3 polarity of solvents. P1 increases with solvent polarity. Laboratory techniques and methods to improve your experimental skills. Web the document lists various solvents and provides their polarity index, viscosity, uv cutoff wavelength, solubility in water, and miscibility. For reverse phase chromatography eluent strength decreases as its polarity increases 2 uv cutoff, the wavelength at which the solvent absorbance in Remember, however, that the color we observe is the complement of the color of the light absorbed. References • classification of the solvent properties of common liquids. Web today, we will explore how polar organic solvents are, and will test what mixes with what. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate dioxane acetone dicholoroethane tetrahydrofuran dicholoromethane chloroform diethylether benzene toluene xylene carbontetrachloride cyclohexane petroleum. Web burdick & jackson solvents are arranged in order of increasing polarity index, a relative measure of the degree of interaction of the solvent with various polar test solutes.
Chemistry Solvent Characteristics CTG Clean

Solvent Polarity Chart A Visual Reference of Charts Chart Master
Solvent Polarity Chart For Tlc
Hplc Solvent Polarity Chart A Visual Reference of Charts Chart Master

Polarity Chart Of Solvents

Polarity Chart Of Solvents

organic solvent polarity chart Bamil
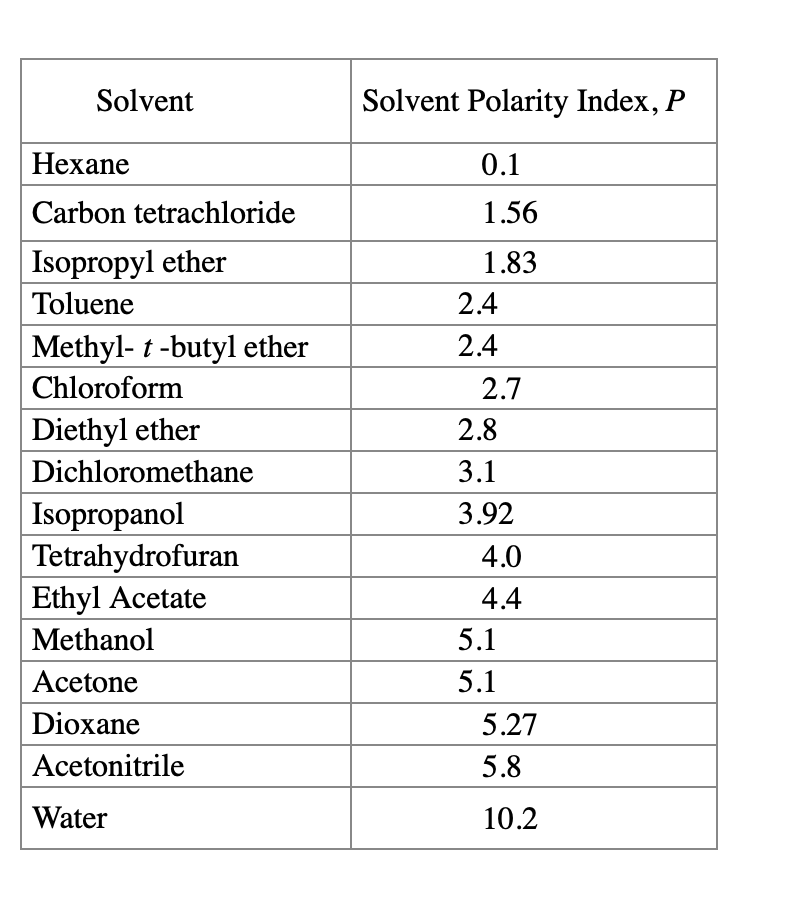
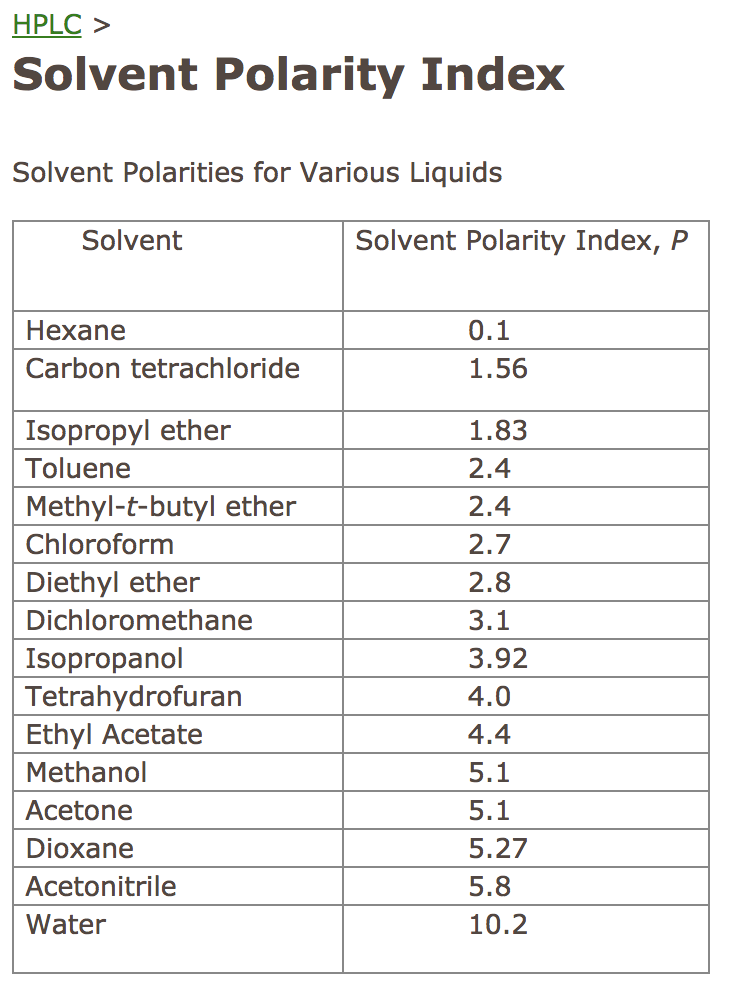
Polarity Of Solvents Chart

Solvent Polarity Chart
Solved Determine the solvent polarity index for each HPLC
Water) Or Quite Nonpolar (E.g.
Web 1 The Polarity Index Is A Measure Of The Relative Polarity Of A Solvent And Is Useful For Identifying Suitable Mobile Phase Solvents.
Web Another Parameter Commonly Manipulated In Crystallization Experiments Is Solvent Polarity.
It Also Includes Charts On Relative Solvent Polarity And Miscibility/Viscosity To Aid In Selecting Suitable Mobile Phase Solvents For Chromatography.
Related Post: