Pka Chart Amino Acids
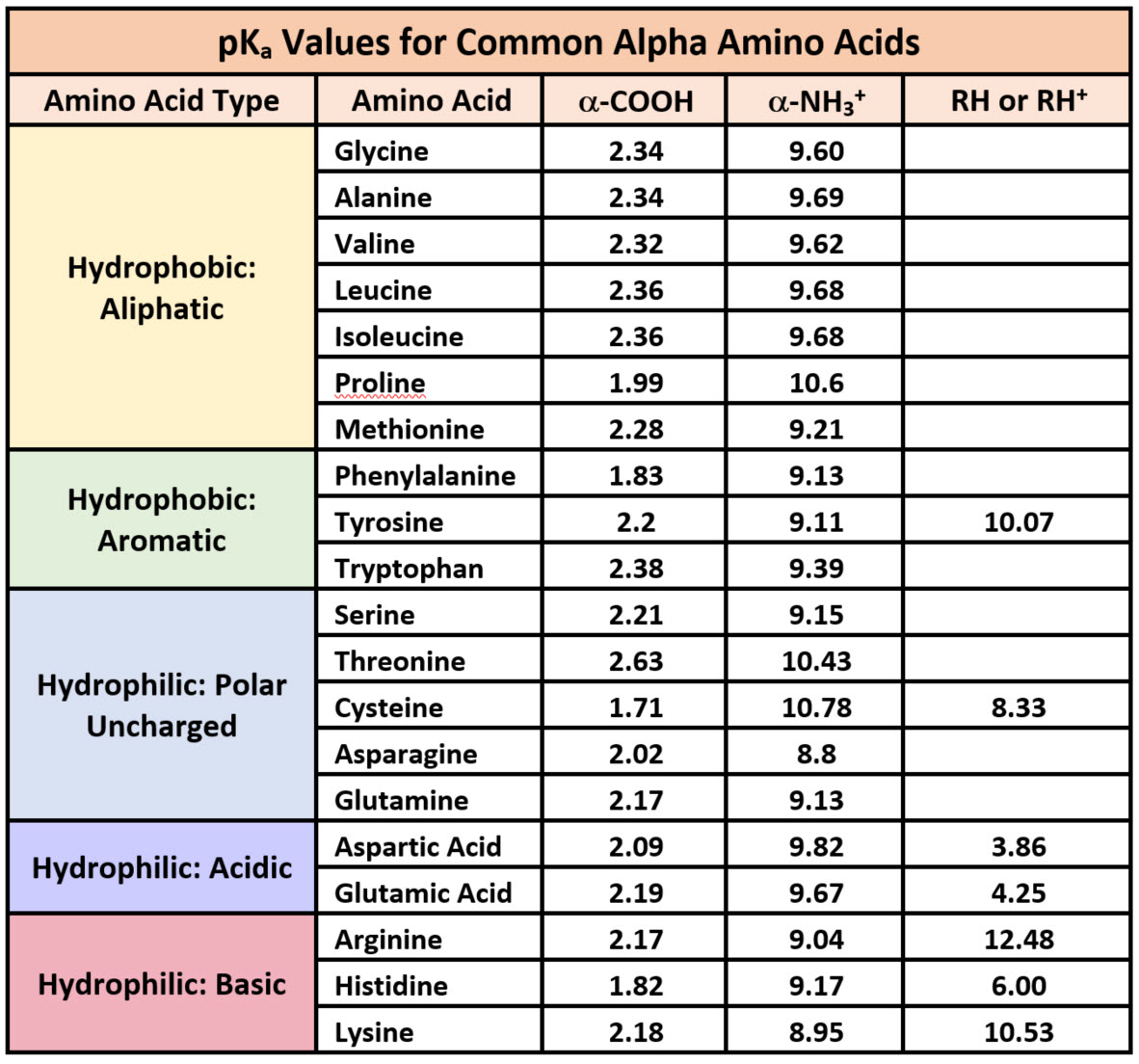
Pka Chart Amino Acids - The ammonium holds the proton more tightly than does the acid. The isoelectric points range from 5.5 to 6.2. Web 20 amino acids and their functions, structures, names, properties, classifications. Calculating isoelectric point pi from pka values. This classification of amino acids has little to do with the structure of amino acids. At neutral ph the amino group is protonated, and the carboxyl group is deprotonated. If a molecule is a base or an acid, depends on their functional groups. The isoelectric point, pi, is the ph at which negative and positive charges are balanced. Refer to chart below to explore structures, properties and types for each of the 20 standard amino acids. Properties of common amino acids. Web pka and electrical properties of amino acids. The isoelectric points range from 5.5 to 6.2. Web 20 amino acids and their functions, structures, names, properties, classifications. A zwitterion is a compound that has no overall charge but that has charge separation within it. Web table of pk a and pi values. Web pka and electrical properties of amino acids. Web for amino acids with basic sidechains, the pi can be calculated by averaging the pk a values of the least acidic groups. For the 13 amino acids with a neutral side chain, pi is the average of pk a1 and pk a2. Web most biochemistry courses will require you to know. Refer to chart below to explore structures, properties and types for each of the 20 standard amino acids. For the four amino acids with either a strongly or weakly acidic side chain, pi is the average of the two lowest pk a values. Web pka and electrical properties of amino acids. Particularly, low molecular weight gelators (lmwgs) consisting of amino. Web for amino acids with basic sidechains, the pi can be calculated by averaging the pk a values of the least acidic groups. Web the isoelectric point of an amino acid is the ph at which the amino acid has a neutral charge. The isoelectric points range from 5.5 to 6.2. You will learn how to calculate the isoelectric point,. This classification of amino acids has little to do with the structure of amino acids. I have been given pka p k a values of an amino group, a carboxyl group and a side chain of cysteine. Web the isoelectric point of an amino acid is the ph at which the amino acid has a neutral charge. Web 20 amino. For the 13 amino acids with a neutral side chain, pi is the average of pk a1 and pk a2. This amino acid cannot be produced by your body and must be obtained through food or through supplements. Properties of common amino acids. At neutral ph the amino group is protonated, and the carboxyl group is deprotonated. The side chains. Web the pka of the acid is near 5, and the pka of the ammonium is near 9. Calculating isoelectric point pi from pka values. A zwitterion is a compound that has no overall charge but that has charge separation within it. Side chains of naturally occurring amino acid embedded in a protein. Web pka and electrical properties of amino. The isoelectric point, pi, is the ph at which negative and positive charges are balanced. Particularly, low molecular weight gelators (lmwgs) consisting of amino acids and short peptides are highly suitable for biological applications owing to their facile synthesis and scalability, as well as their biocompatibility,. We will also discuss zwitterions, or the forms of amino acids that dominate at. Particularly, low molecular weight gelators (lmwgs) consisting of amino acids and short peptides are highly suitable for biological applications owing to their facile synthesis and scalability, as well as their biocompatibility,. It represents the negative logarithm of the acid dissociation constant (ka), which indicates the tendency of a molecule to donate or accept protons in a chemical reaction. Web table. At neutral ph the amino group is protonated, and the carboxyl group is deprotonated. These tables are compiled in pdf files below. Web the pka is a measure of the strength of an acid, i.e., the lower the pk a stronger the acid. Web amino acids reference chart. I have been given pka p k a values of an amino. Web 20 amino acids and their functions, structures, names, properties, classifications. Web amino acids reference chart. Web the pka is a measure of the strength of an acid, i.e., the lower the pk a stronger the acid. 3 pkx is the negative of the logarithm of the dissociation constant for any other group in the molecule. A zwitterion is a compound that has no overall charge but that has charge separation within it. It represents the negative logarithm of the acid dissociation constant (ka), which indicates the tendency of a molecule to donate or accept protons in a chemical reaction. For the 13 amino acids with a neutral side chain, pi is the average of pk a1 and pk a2. Asked 10 years, 8 months ago. The isoelectric point, pi, is the ph at which negative and positive charges are balanced. Amino acid pka c pka n pka r pi; That is a daunting task for 20 amino acids. Web table of pk a and pi values. The proton stays on the nitrogen. Web amino acid pka and pi values. The isoelectric points range from 5.5 to 6.2. Web the r group, which differs for each amino acid, will determine its structure, polarity and ph.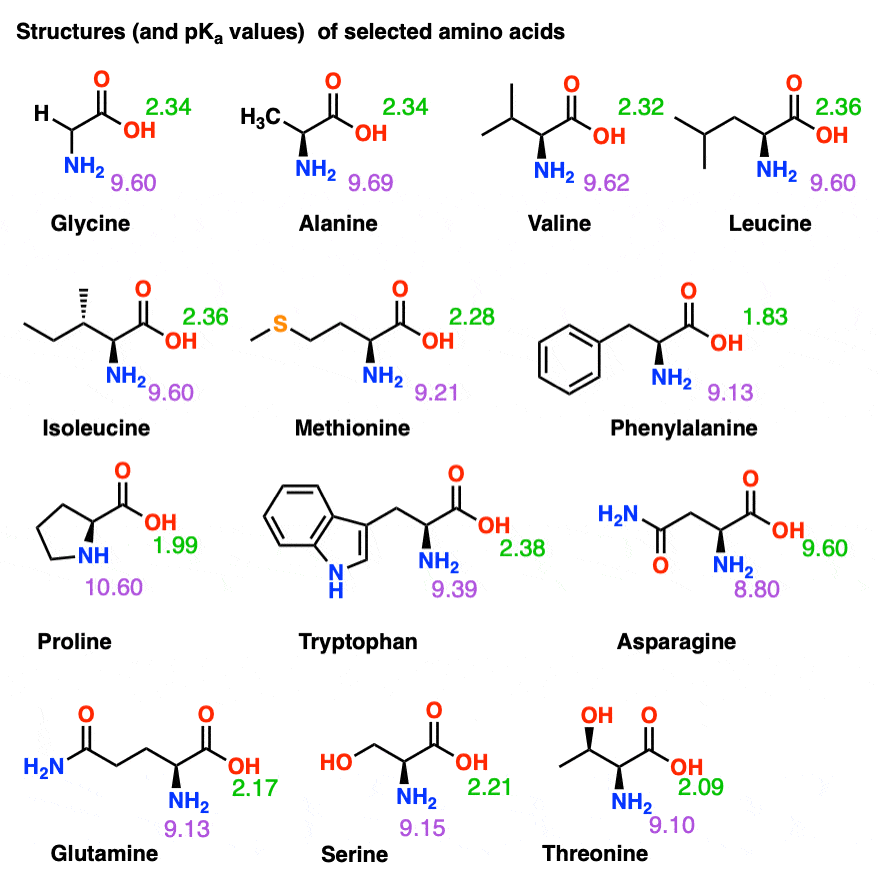
Isoelectric Points of Amino Acids (and How To Calculate Them) Master
3.1 Amino Acids and Peptides Biology LibreTexts
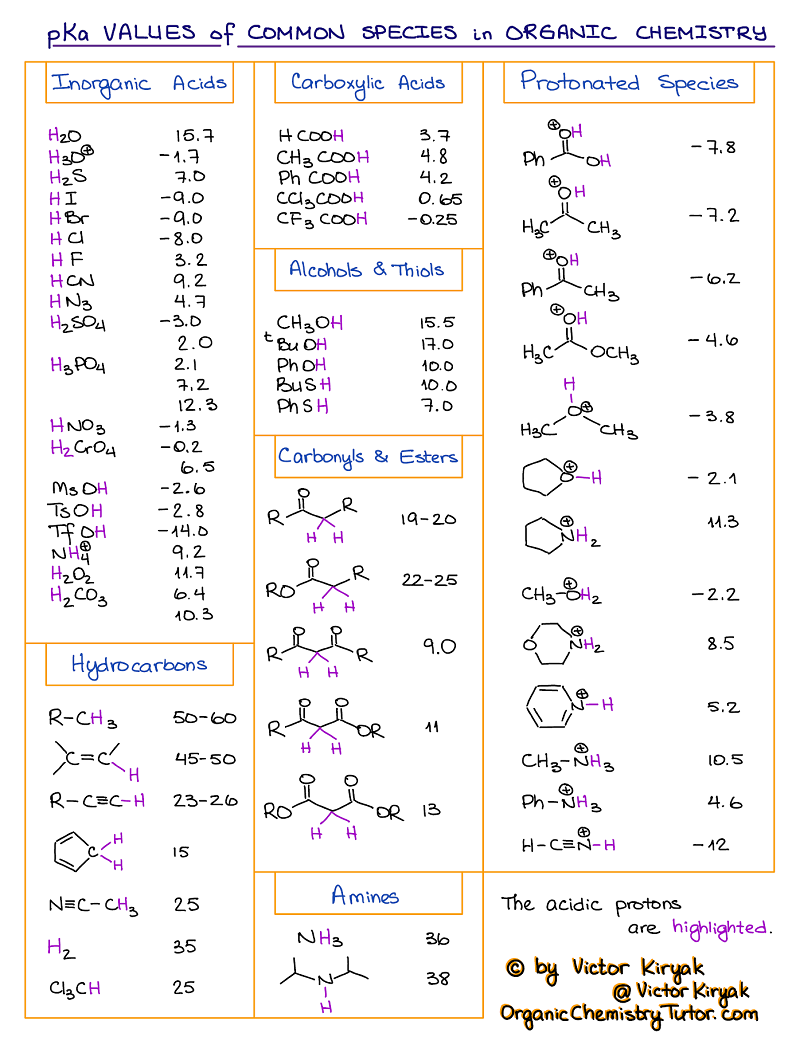
pKa Table and How to Use It — Organic Chemistry Tutor

Amino Acid Chart Pka
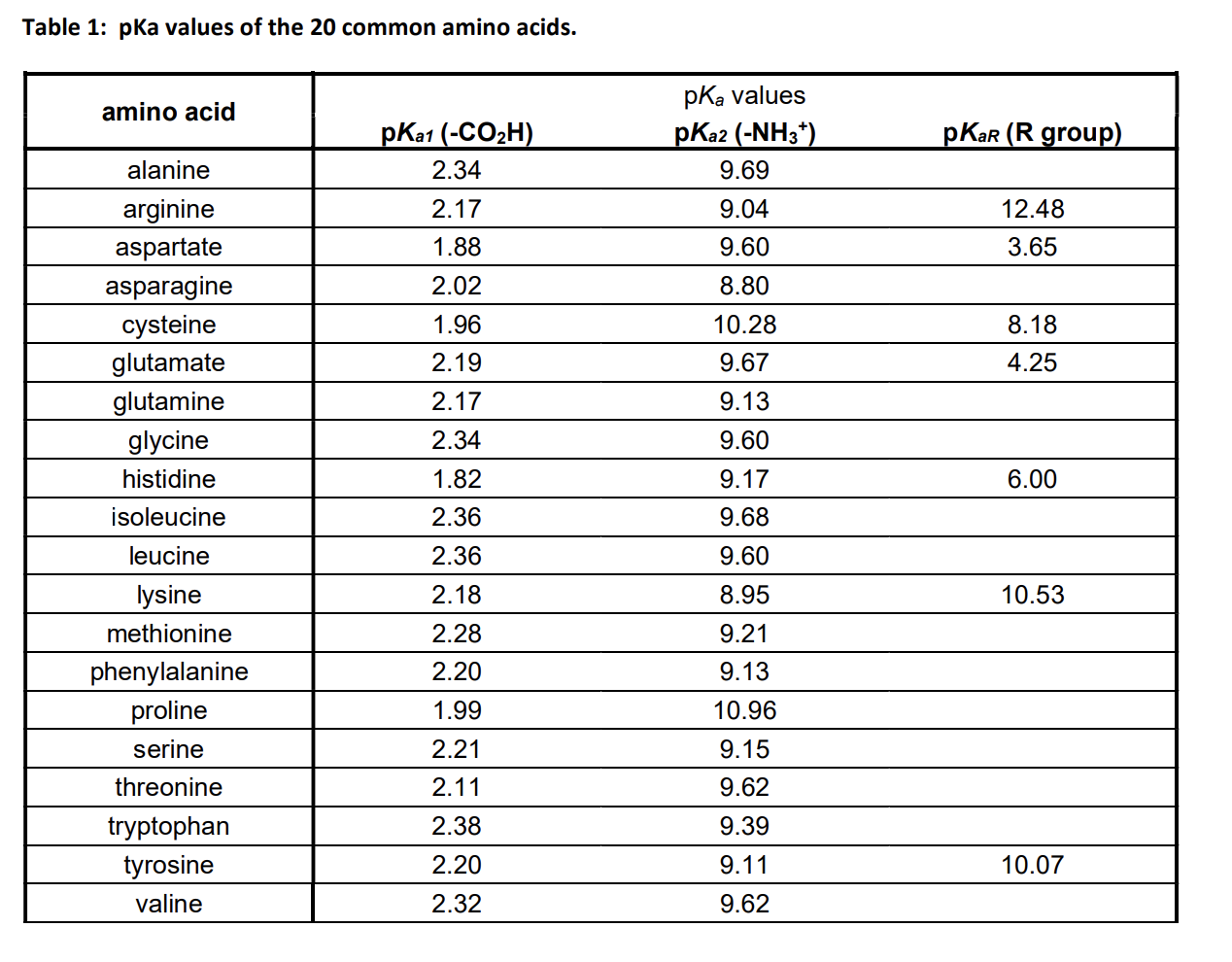
Solved Table 1 pKa values of the 20 common amino acids.
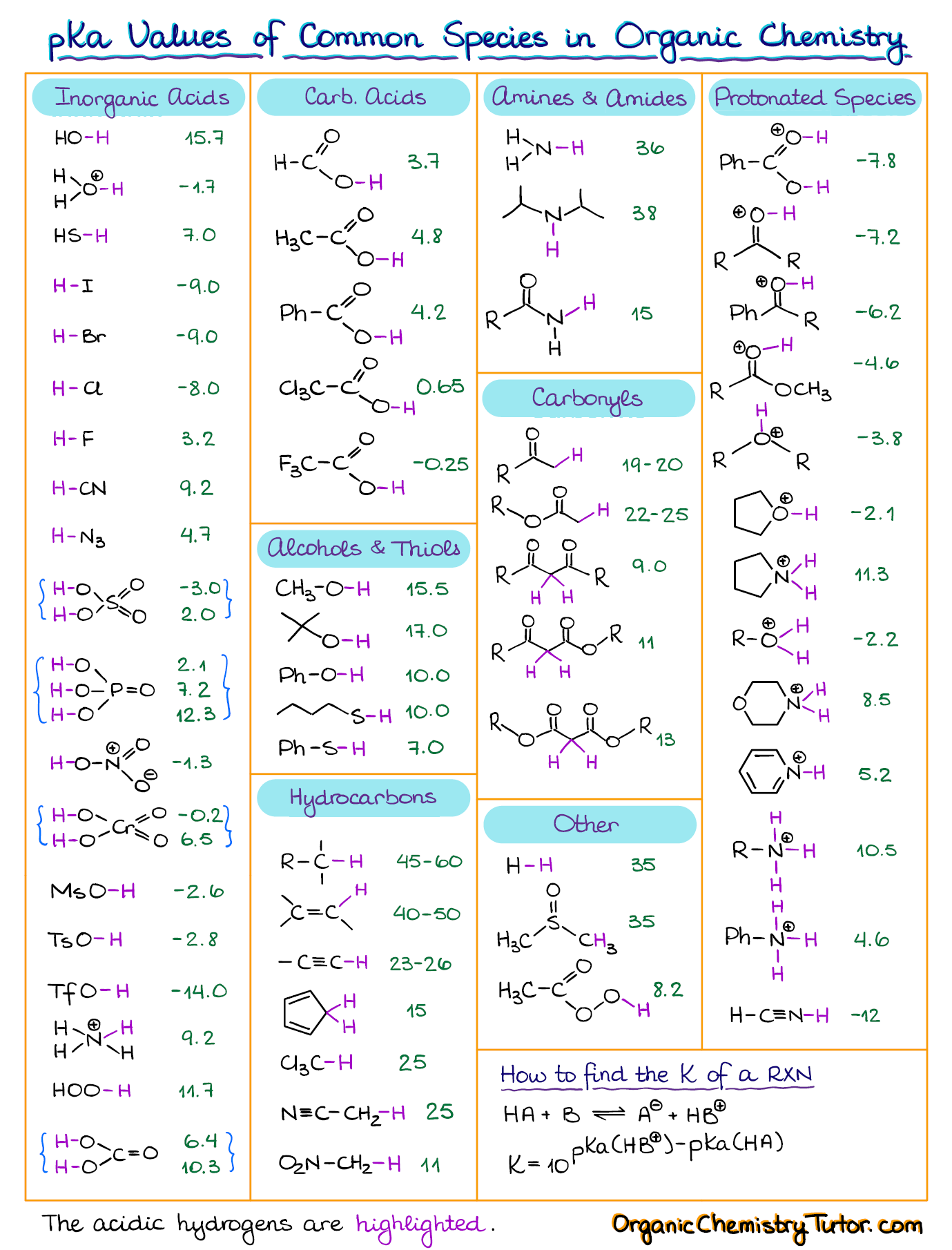
AcidBase Equilibrium Part 1 How to Use the pKa Table — Organic
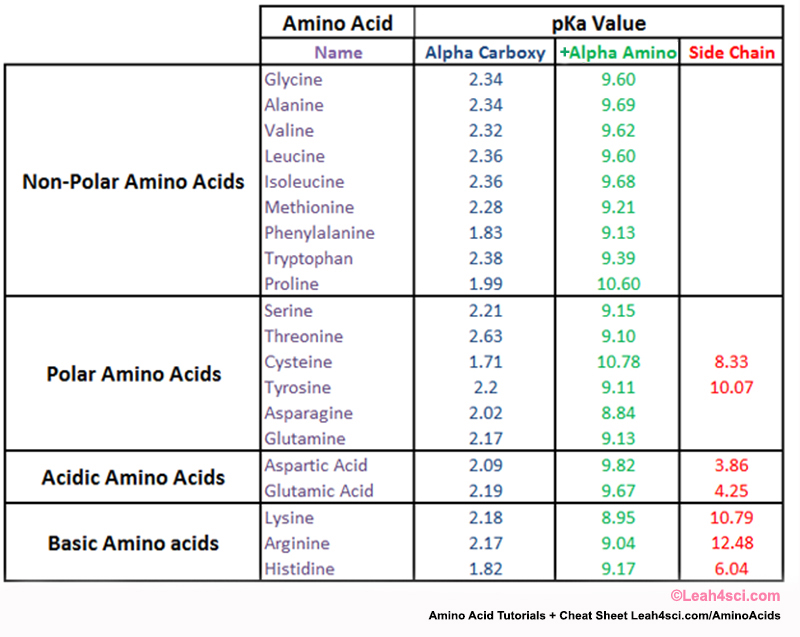
Amino Acid Charge in Zwitterions and Isoelectric Point MCAT Tutorial
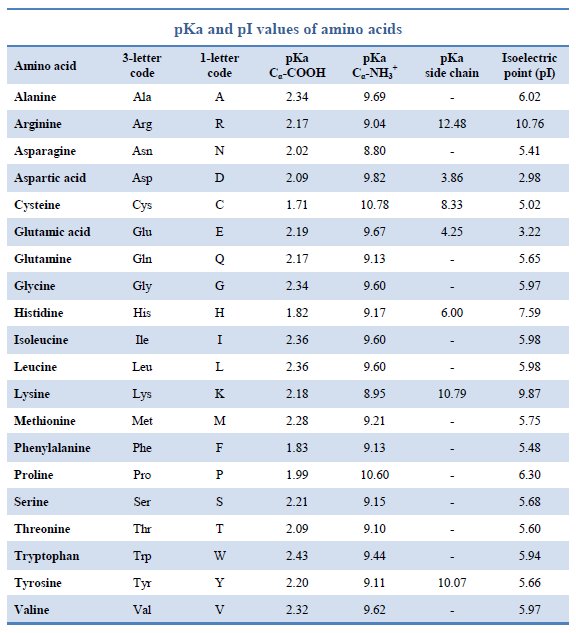
Amino acid properties
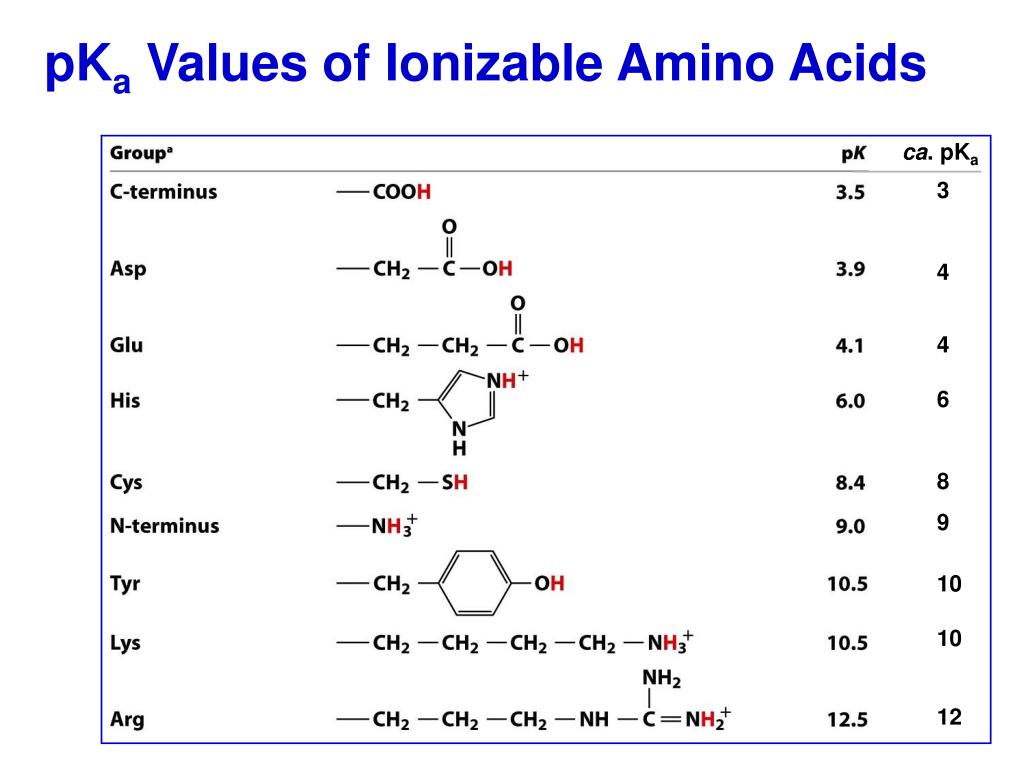
PPT Proteins Amino Acid Chains PowerPoint Presentation, free
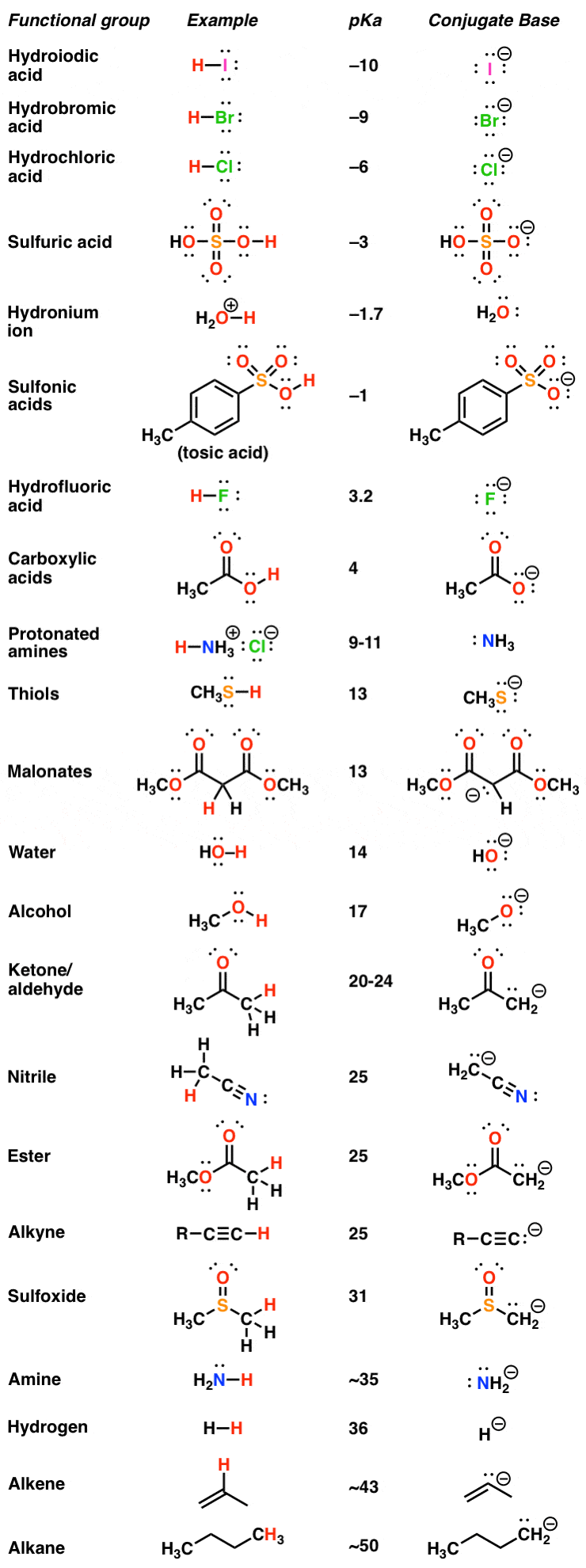
AcidBase Reactions Introducing Ka and pKa Master Organic Chemistry
At Neutral Ph The Amino Group Is Protonated, And The Carboxyl Group Is Deprotonated.
Modified 6 Years, 1 Month Ago.
Refer To Chart Below To Explore Structures, Properties And Types For Each Of The 20 Standard Amino Acids.
Web Pka And Electrical Properties Of Amino Acids.
Related Post: