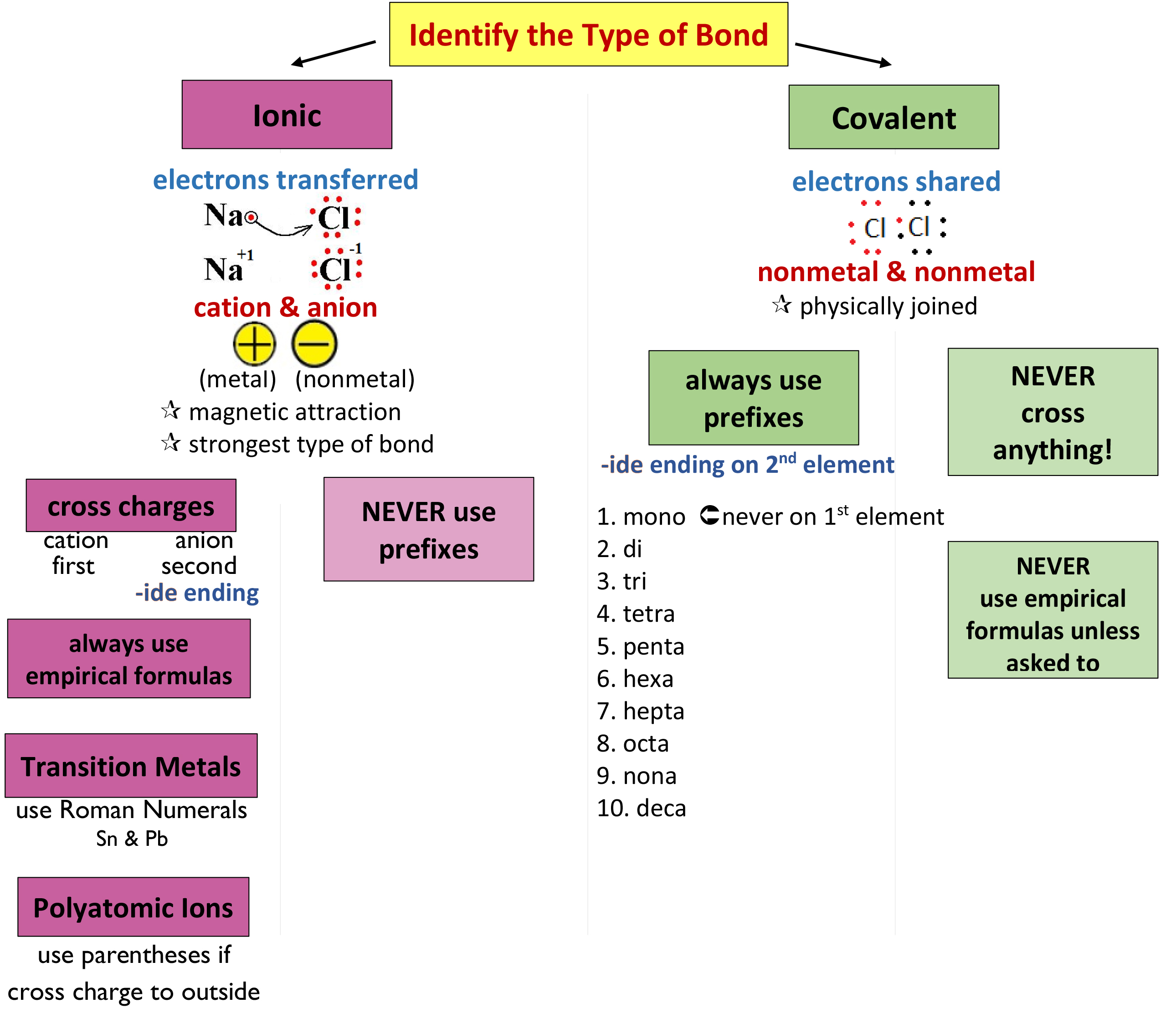
Naming Compounds Chart
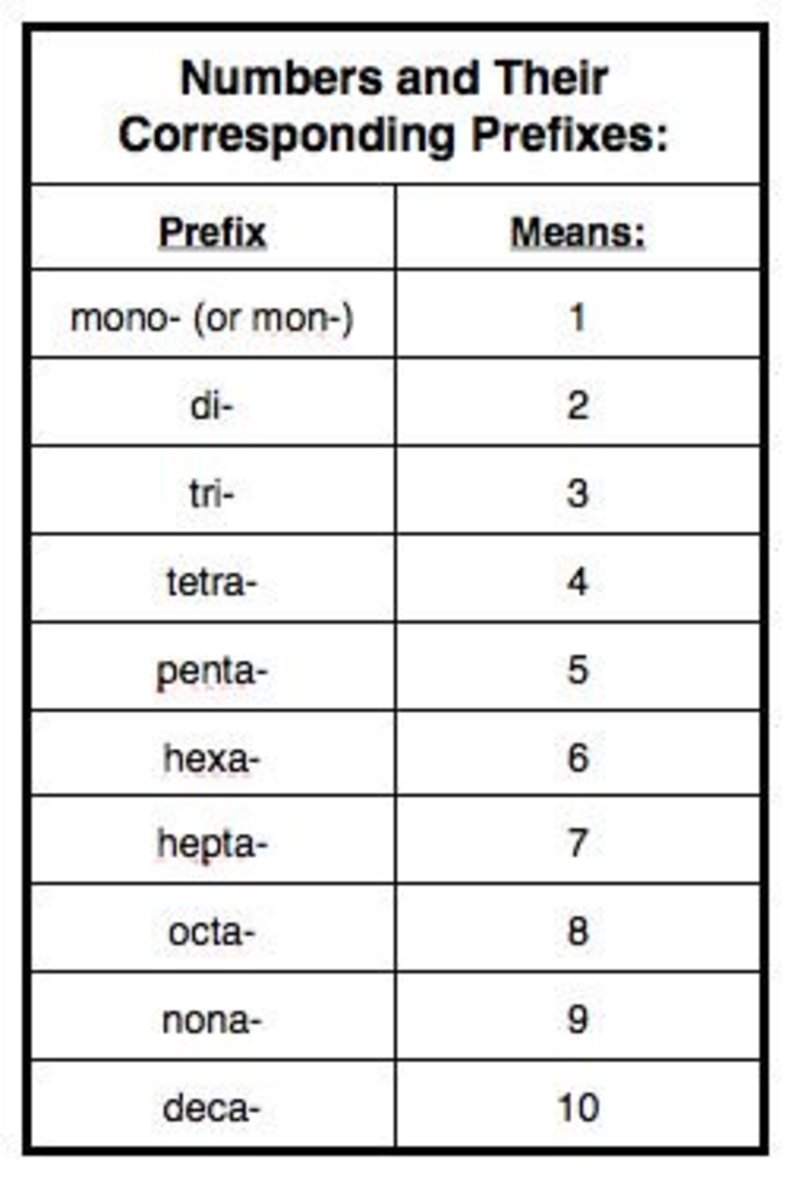
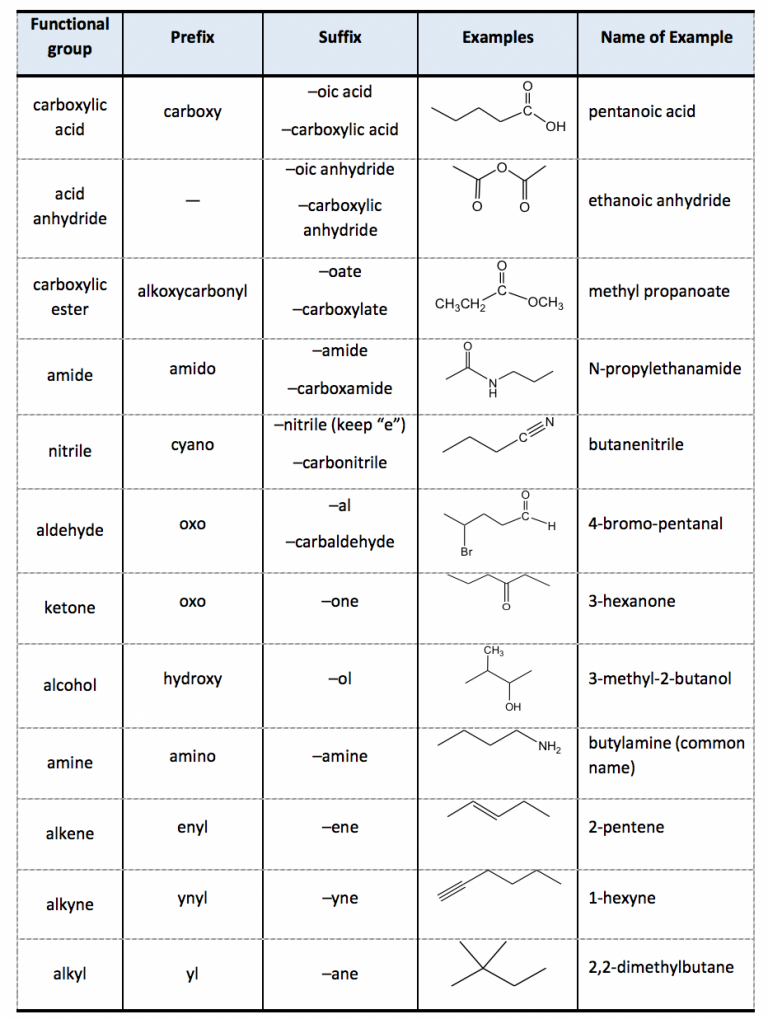
Naming Compounds Chart - Look up the name of the nonmetal on the periodic table. Roman numerals are used to indicate the charges of the multivalent transition metal. Today we often use chemical formulas, such as nacl, c 12 h 22 o 11, and co (nh 3) 6 (clo 4) 3 , to describe chemical compounds. Web derive names for common types of inorganic compounds using a systematic approach. If the compound ends with a polyatomic ion, it will not end in ide. do not change the name of the polyatomic ion. A binary compound is one that consists of only two elements. Web learn how to name positive ions (cations), negative ions (anions), and ionic compounds involving main group elements. Web nomenclature, a collection of rules for naming things, is important in science and in many other situations. Cation is named first before the anion. Nomenclature, a collection of rules for naming things, is important in science and in many other situations. This includes an introduction to the iupac system of nomenclature, the steps to follow when naming an organic compound, and some practice examples. The corresponding modern letter prefixed with a dollar. Web naming organic compounds according to the iu {ac system requires up to four pieces of information. For example, [latex]\text{naf}[/latex] is also known as sodium fluoride. The primary function. Web to name a compound, the cation name and the anion named are added together. The appropriate unicode character e.g. The primary function of chemical nomenclature is to ensure that a spoken or written chemical name leaves no ambiguity concerning which chemical compound the name refers to—each chemical name should. Binary compound (metal/nonmetal) with fixed charge cation. Web nomenclature, a. For example, [latex]\text{naf}[/latex] is also known as sodium fluoride. This includes an introduction to the iupac system of nomenclature, the steps to follow when naming an organic compound, and some practice examples. The chart will tell you how to name the compound. Compounds end in ide. mono can be left off of the first element but not the second element.. The acid and base chart is a reference table designed to make determining the strength of acids and. Roman numerals are used to indicate the charges of the multivalent transition metal. Cation is named first before the anion. Web the system used for naming chemical substances depends on the nature of the molecular units making up the compound. To describe. Today we often use chemical formulas, such as nacl, c 12 h 22 o 11, and co (nh 3) 6 (clo 4) 3 , to describe chemical compounds. The primary function of chemical nomenclature is to ensure that a spoken or written chemical name leaves no ambiguity concerning which chemical compound the name refers to—each chemical name should. Web look. When naming a chemical compound, it is important to know the number of atoms in each element. Web this module describes an approach that is used to name simple ionic and molecular compounds, such as nacl, caco 3, and n 2 o 4. Recognize & prioritize the functional group (s) present. Greek characters greek characters may be specified by: Web. Use this acids and bases chart to find the relative strength of the most common acids and bases. Web rules for naming covalent compounds. Web this module describes an approach that is used to name simple ionic and molecular compounds, such as nacl, caco 3, and n 2 o 4. A binary compound is one that consists of only two. Different rules apply to each. The appropriate unicode character e.g. Web look up the name of the polyatomic ion (selected ions on the reverse of this chart). Web to name a compound, the cation name and the anion named are added together. A binary compound is one that consists of only two elements. The polyatomic ion (positive ion) name is written first, followed by the name of the nonmetal. When naming a chemical compound, it is important to know the number of atoms in each element. First, name the nonmetal furthest to the left and bottom of the periodic table by its element name. The romanised name for the letter surrounded by dots. Different rules apply to each. Web nomenclature is the process of naming chemical compounds so that they can be easily identified as separate chemicals. If the compound ends with a polyatomic ion, it will not end in ide. do not change the name of the polyatomic ion. For example, [latex]\text{naf}[/latex] is also known as sodium fluoride. A binary compound is. Compounds end in ide. mono can be left off of the first element but not the second element. Web 4 steps to naming compounds in chemistry nomenclature | by ernest wolfe | countdown.education | medium. In this section, we discuss. Binary compound (metal/nonmetal) with variable charge cation: To describe the composition of a chemical compound. The appropriate unicode character e.g. Web tutorials and problem sets. Chemblogmom) view full chart here. The corresponding modern letter prefixed with a dollar. Web nomenclature, a collection of rules for naming things, is important in science and in many other situations. These are usually either ions or molecules; Web rules for naming covalent compounds. Check the chart below (no prefixes!) use the names on the chart. Web in this tutorial, you will learn all about the iupac naming of organic compounds. Web naming organic compounds according to the iu {ac system requires up to four pieces of information. Describe how to name binary covalent compounds including acids and oxyacids.Chemistry Naming Molecular Compounds

Naming Compounds Griger Science
07 Nomenclature Mrs. Cook's Chemistry Class
Atoms & Compounds

Organic Chemistry Nomenclature Chart
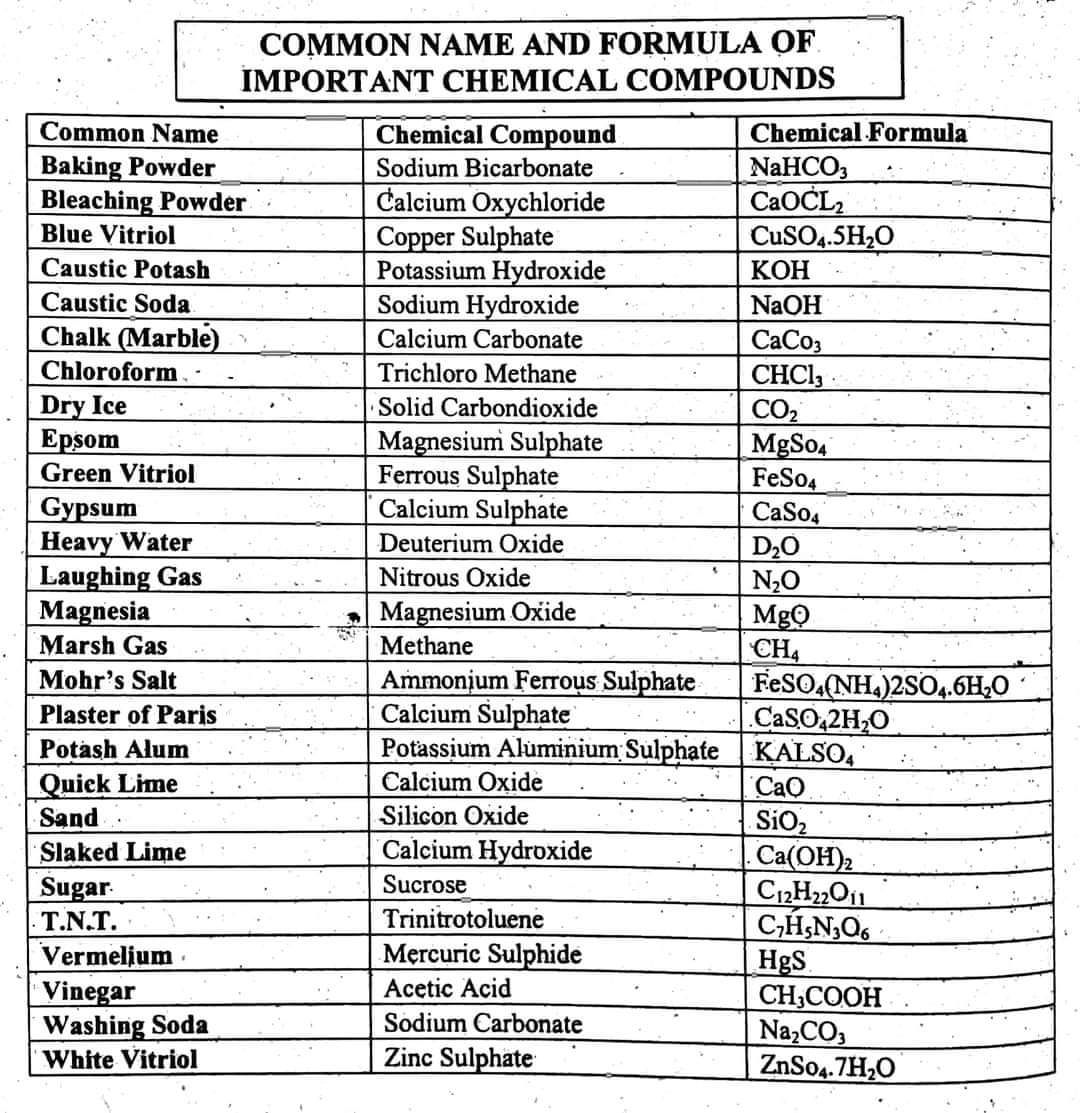
Formula of Important Chemical Compounds
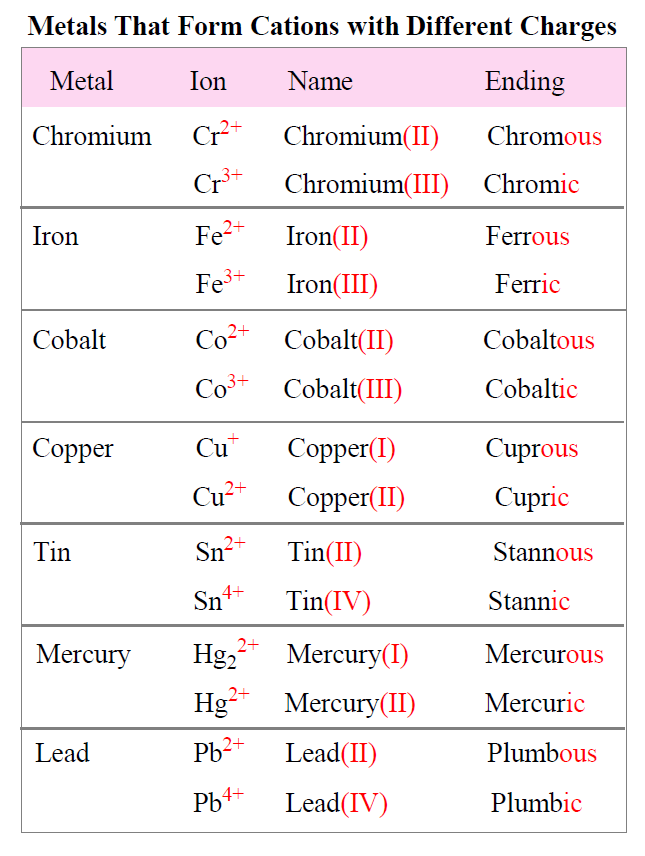
Naming Ionic Compounds Chemistry Steps
Chemistry Nomenclature Chart
Chemistry Nomenclature Chart

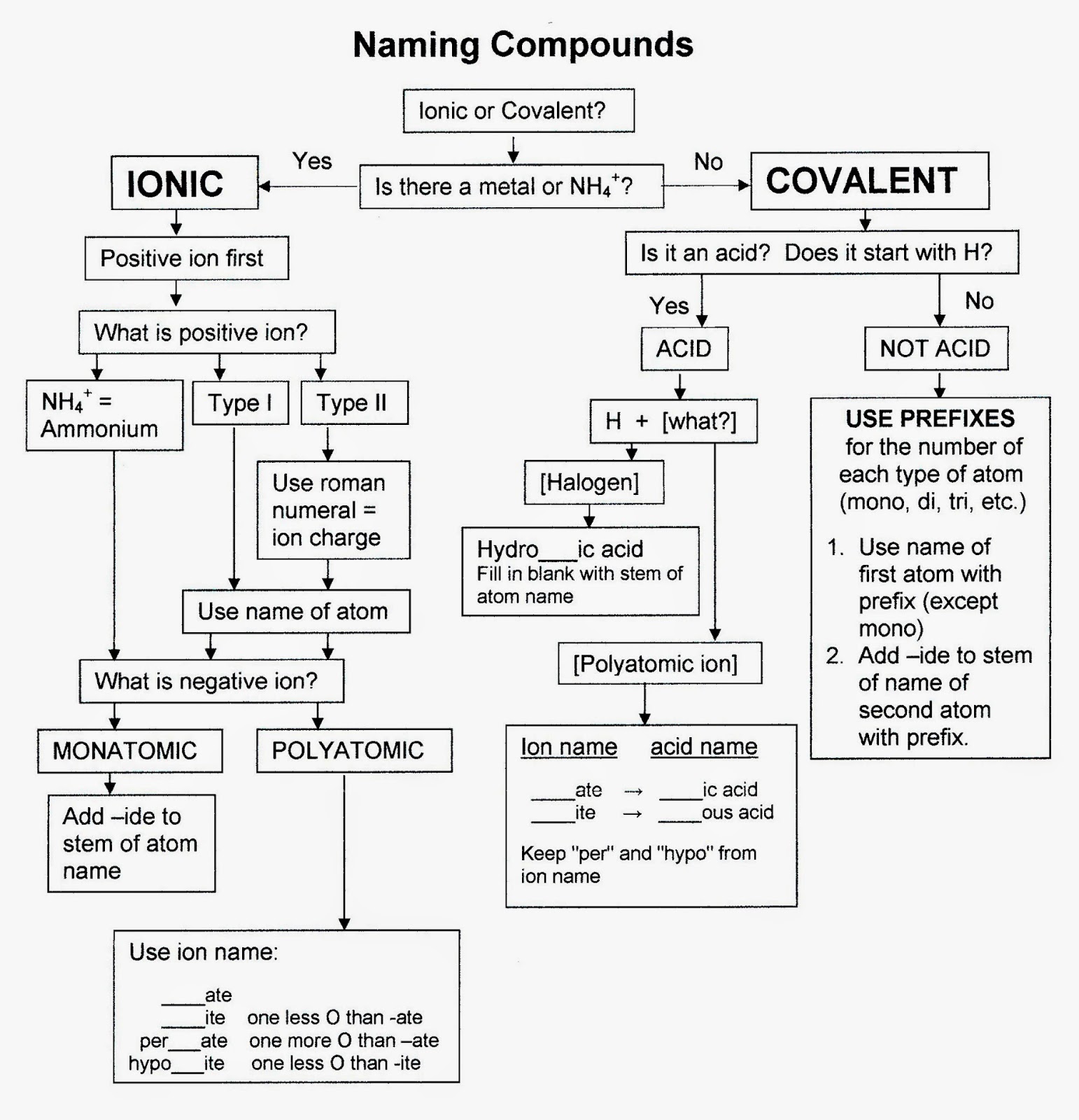
Naming Compounds Flowchart Chemistry Guide
This Module Describes An Approach That Is Used To Name Simple Ionic And Molecular Compounds, Such As Nacl, Caco 3, And N 2 O 4.
To Name Covalent Compounds That Contain Up To Three Elements.
Greek Characters Greek Characters May Be Specified By:
Web Long Before Chemists Knew The Formulas For Chemical Compounds, They Developed A System Of Nomenclature That Gave Each Compound A Unique Name.
Related Post:
.PNG)