Medical Device Design
Medical Device Design - Web this comprehensive guide aims to unravel the intricacies of medical device design and development, providing valuable insights for those navigating this complex terrain. Devicelab has refined a model of medical device development that captures both regulatory imperatives and best practices in business and engineering. Medical devices range from simple tools like bandages to complex implantable devices like pacemakers. Web a medical device development model. Web medical device design is the process of conceptualizing, developing, and refining diagnostic equipment, preventative devices, or instruments used to monitor or treat medical conditions. As medical device designers, our team of experts ensures that we follow the latest regulations and follow best practices. Includes new case studies in the areas of classifying medical devices, the design process, quality, labeling, instructions for use, and more The global markets for medical devices is growing fast — by $7 billion a year in the united states alone, by one estimate. Web in this article, we will focus more on medical device design, design controls, and compliance. By now you know that for market entry your medical device needs to qualify certain regulatory requirements and standards. Web this comprehensive guide aims to unravel the intricacies of medical device design and development, providing valuable insights for those navigating this complex terrain. Devicelab has refined a model of medical device development that captures both regulatory imperatives and best practices in business and engineering. As medical device designers, our team of experts ensures that we follow the latest regulations. Iec & iso regulations and compliance. Web medical device design is the process of conceptualizing, developing, and refining diagnostic equipment, preventative devices, or instruments used to monitor or treat medical conditions. Web this comprehensive guide aims to unravel the intricacies of medical device design and development, providing valuable insights for those navigating this complex terrain. Each year we bring together. Web a medical device development model. Medical devices range from simple tools like bandages to complex implantable devices like pacemakers. Iec & iso regulations and compliance. Devicelab has refined a model of medical device development that captures both regulatory imperatives and best practices in business and engineering. The global markets for medical devices is growing fast — by $7 billion. The guide will walk you through fundamentals of medical device design and development, provide information on processes, and; By now you know that for market entry your medical device needs to qualify certain regulatory requirements and standards. Each year we bring together clinicians, industry partners and students, faculty and staff to prototype new healthcare technologies that address challenges brought to. The global markets for medical devices is growing fast — by $7 billion a year in the united states alone, by one estimate. By now you know that for market entry your medical device needs to qualify certain regulatory requirements and standards. Web a comprehensive guide for medtech professionals to design and develop effective medical devices. Web this comprehensive guide. Medical devices range from simple tools like bandages to complex implantable devices like pacemakers. Devicelab has refined a model of medical device development that captures both regulatory imperatives and best practices in business and engineering. Includes new case studies in the areas of classifying medical devices, the design process, quality, labeling, instructions for use, and more By now you know. Web medical device design is the process of conceptualizing, developing, and refining diagnostic equipment, preventative devices, or instruments used to monitor or treat medical conditions. Each year we bring together clinicians, industry partners and students, faculty and staff to prototype new healthcare technologies that address challenges brought to us by clinicians and industry partners. The guide will walk you through. Devicelab has refined a model of medical device development that captures both regulatory imperatives and best practices in business and engineering. Web a comprehensive guide for medtech professionals to design and develop effective medical devices. Web this comprehensive guide aims to unravel the intricacies of medical device design and development, providing valuable insights for those navigating this complex terrain. Web. Web this comprehensive guide aims to unravel the intricacies of medical device design and development, providing valuable insights for those navigating this complex terrain. Web medical device design is the process of designing a device intended to be used for medical purposes. Iec & iso regulations and compliance. Help you plan your next device. Each year we bring together clinicians,. Iec & iso regulations and compliance. The global markets for medical devices is growing fast — by $7 billion a year in the united states alone, by one estimate. Medical devices range from simple tools like bandages to complex implantable devices like pacemakers. By now you know that for market entry your medical device needs to qualify certain regulatory requirements. As medical device designers, our team of experts ensures that we follow the latest regulations and follow best practices. This blog series presents that model and explores how it accomplishes the goals we set for it. Devicelab has refined a model of medical device development that captures both regulatory imperatives and best practices in business and engineering. The global markets for medical devices is growing fast — by $7 billion a year in the united states alone, by one estimate. Help you plan your next device. Web medical device design is the process of designing a device intended to be used for medical purposes. Web medical device design is the process of conceptualizing, developing, and refining diagnostic equipment, preventative devices, or instruments used to monitor or treat medical conditions. Web in this article, we will focus more on medical device design, design controls, and compliance. Web a comprehensive guide for medtech professionals to design and develop effective medical devices. Web provides a reference to standards and regulations that have been updated, including iso 13485:2016, fda regulations and the european medical device regulation; Medical devices range from simple tools like bandages to complex implantable devices like pacemakers. Iec & iso regulations and compliance. By now you know that for market entry your medical device needs to qualify certain regulatory requirements and standards. The guide will walk you through fundamentals of medical device design and development, provide information on processes, and;
Medical Device Design on Behance

Improving Medical Device Design with Simulation Technology Javelin 3D

Medical Device Design Guide Phase 2 Research

How To Design a Medical Device? Creanova

Medical Device Design on Behance
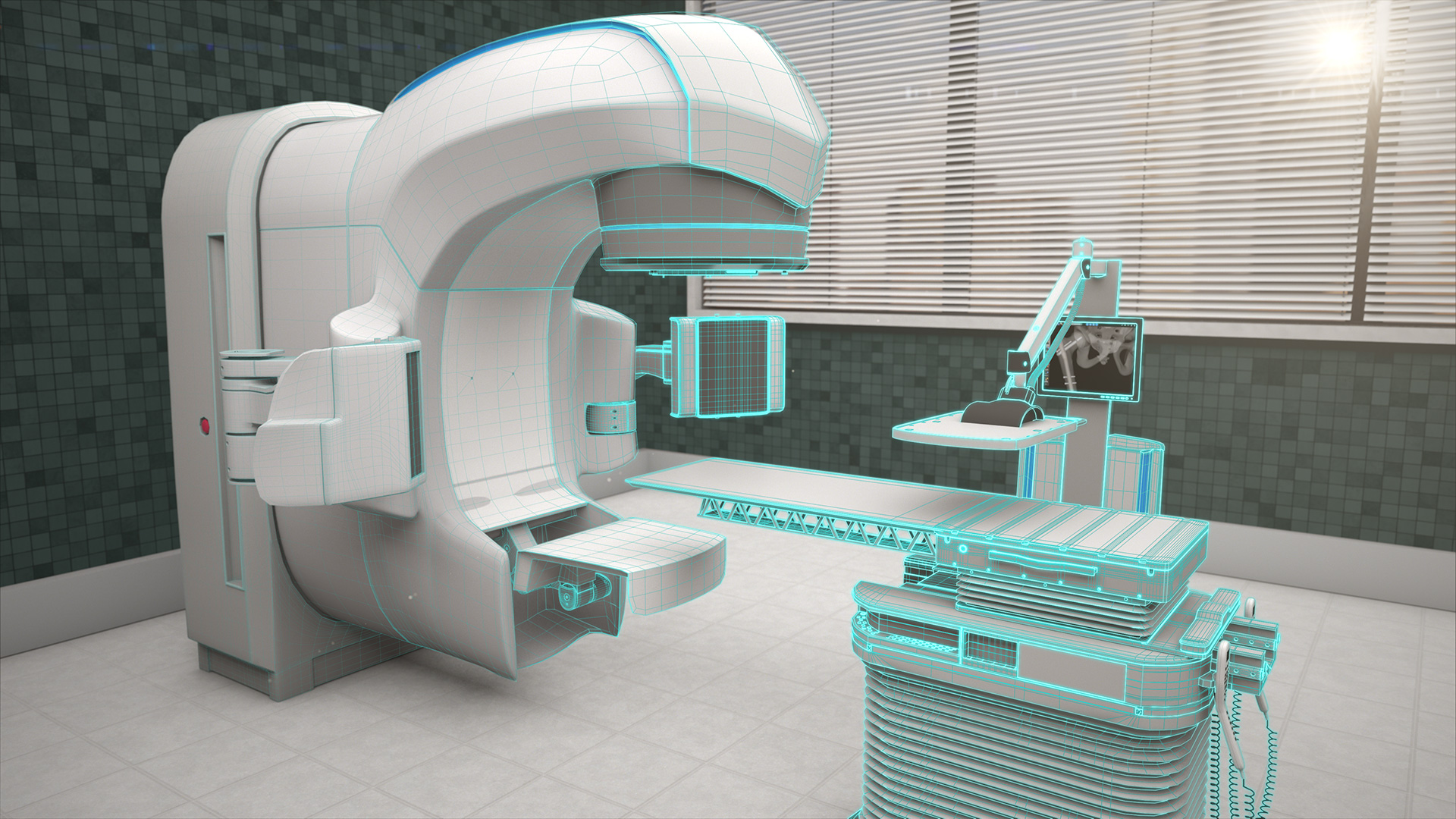
An Intelligent Approach to Design Control for Medical Devices Siemens
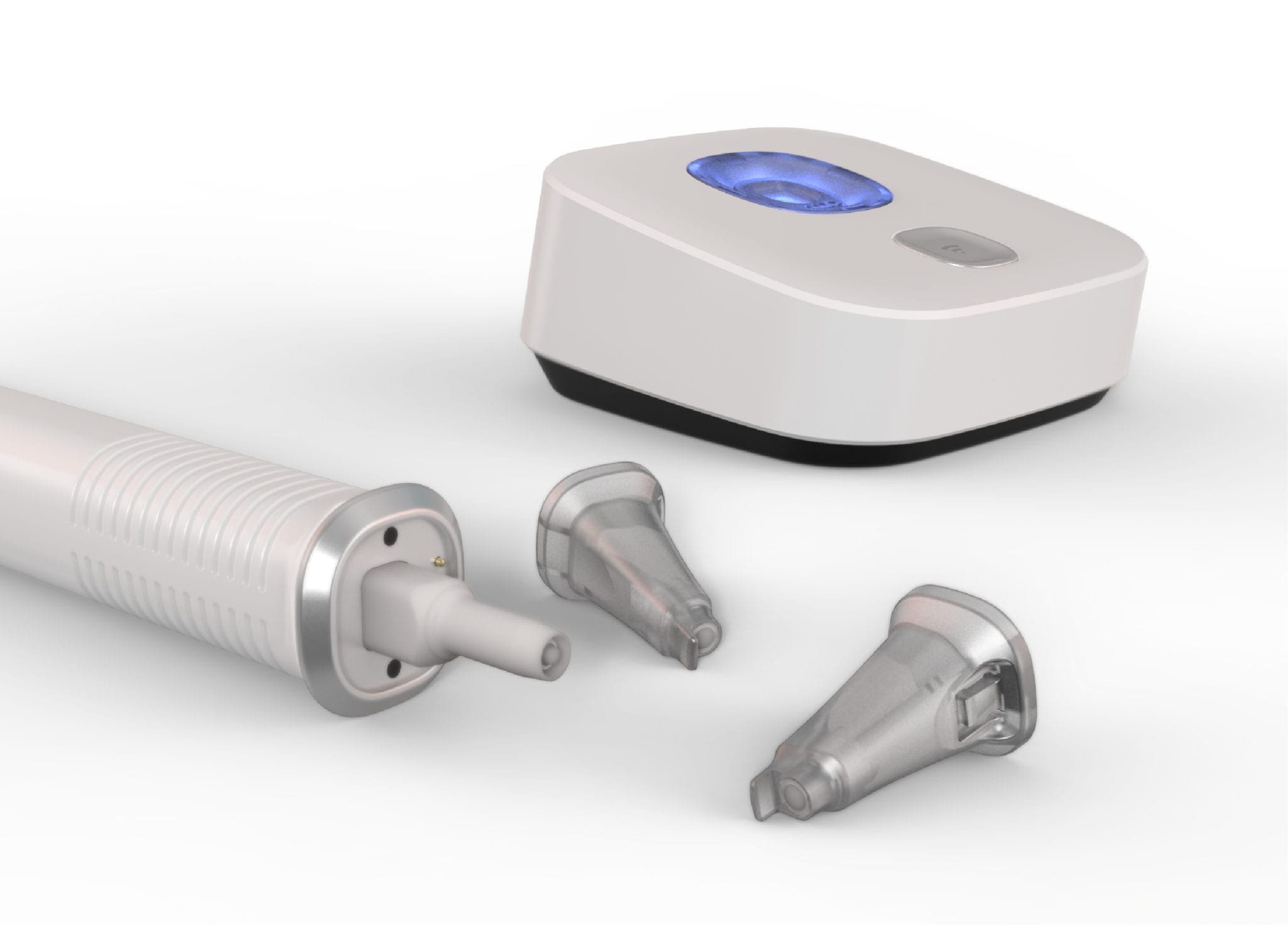
Who designs medical devices? Our medical device product design guide.

Medical Device Design on Behance

Medical Device Design on Behance
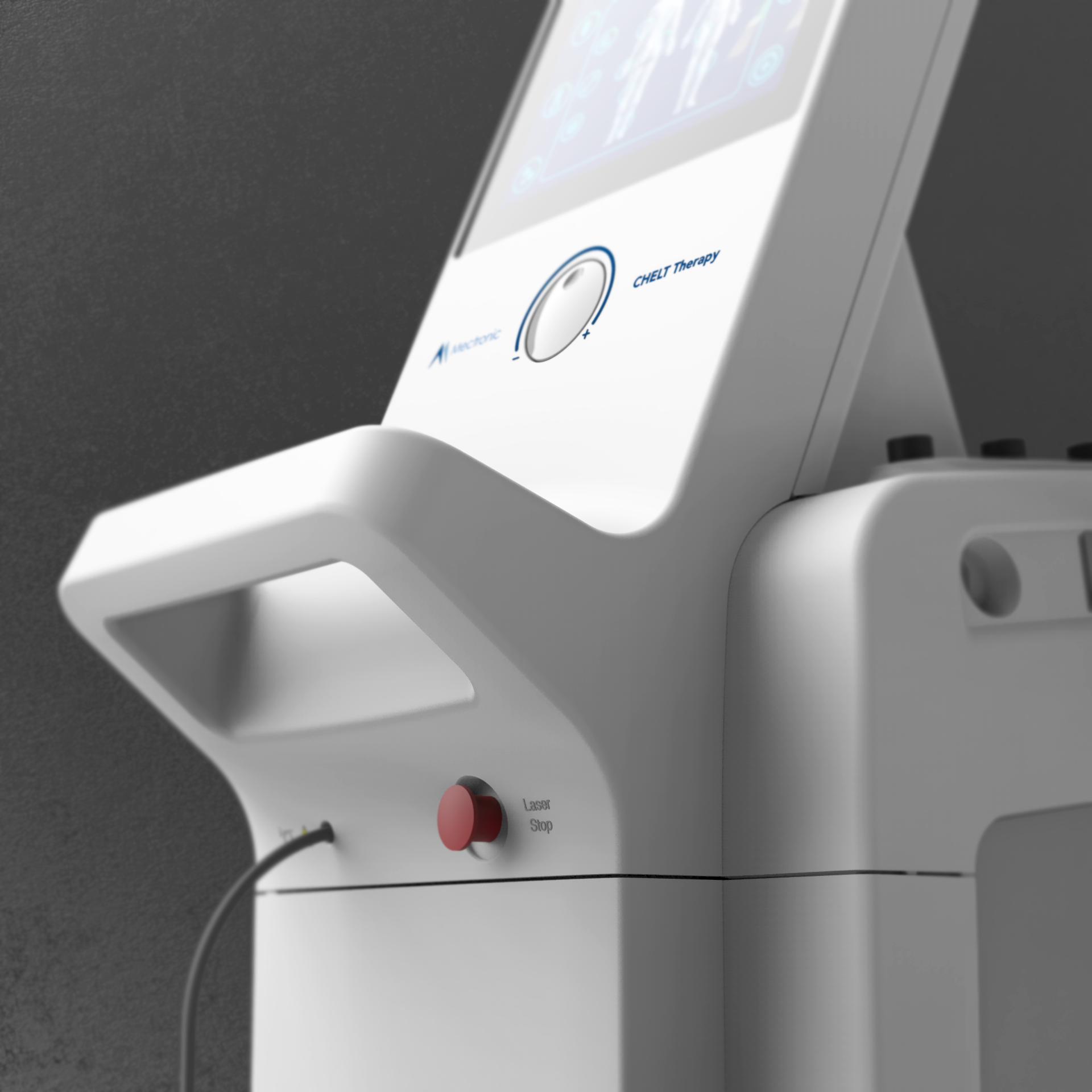
How to create a good design for your medical device Creanova
Web A Medical Device Development Model.
Each Year We Bring Together Clinicians, Industry Partners And Students, Faculty And Staff To Prototype New Healthcare Technologies That Address Challenges Brought To Us By Clinicians And Industry Partners.
Includes New Case Studies In The Areas Of Classifying Medical Devices, The Design Process, Quality, Labeling, Instructions For Use, And More
Web This Comprehensive Guide Aims To Unravel The Intricacies Of Medical Device Design And Development, Providing Valuable Insights For Those Navigating This Complex Terrain.
Related Post: