Isotope Drawing
Isotope Drawing - Atoms of the same element with different mass numbers are called isotopes. The relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring sample of an element. Web how to draw an isotope. Web what are isotopes, and why do i need to know about them? They share the same atomic number and therefore the same number of. Web an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in the periodic table and nearly identical chemical behavior but with different atomic masses and physical properties. Web high school students need concrete methods to get them engaged in learning about isotopes and atomic structure. Web new research using apollo mission samples has determined that the impact of meteorites on the lunar surface is primarily responsible for generating the moon's thin and nebulous atmosphere. Isotopes have different atomic masses. Atoms that have the same number of protons but different numbers of neutrons are known as isotopes. Every chemical element has one or more isotopes. Thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. Web an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in the periodic table and nearly identical. Web drawing atomic structure requires only a simple understanding of the components of atomic structure. Web build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Each isotope comprehends of unique properties. These resources will explore these questions and more, including how the new atomic weight intervals came to be, and how. Deuterium naturally occurs in the earth’s oceans as deuterium oxide or heavy water. Web the term isotope is derived from the greek roots isos (ἴσος equal) and topos (τόπος place), meaning the same place; 4 h to 7 h are nuclei isotopes that are incorporated in the laboratory. Web build an atom out of protons, neutrons, and electrons, and see. Web isotopes are members of a family of an element that all have the same number of protons but different numbers of neutrons. Web the specific ratio of light to heavy isotopes that remain in the soil, for both potassium and rubidium, should then reveal the main process contributing to the lunar atmosphere's origins. Atoms that have the same number. Web new research using apollo mission samples has determined that the impact of meteorites on the lunar surface is primarily responsible for generating the moon's thin and nebulous atmosphere. Additionally, n = a −z. Web what are the two main types of isotopes? Web drawing atomic structure requires only a simple understanding of the components of atomic structure. Web an. Atoms that have the same number of protons but different numbers of neutrons are known as isotopes. Web how to draw an isotope. These isotopes are in common used to date. Web here are a few examples of isotopes: Web high school students need concrete methods to get them engaged in learning about isotopes and atomic structure. Deuterium naturally occurs in the earth’s oceans as deuterium oxide or heavy water. Atoms of the same element with different mass numbers are called isotopes. Thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. Web isotope notation, also known as nuclear notation, is important because it allows. Web explore the concepts of isotopes and atomic mass with this interactive simulation. Web how to draw an isotope. Web the specific ratio of light to heavy isotopes that remain in the soil, for both potassium and rubidium, should then reveal the main process contributing to the lunar atmosphere's origins. 4 h to 7 h are nuclei isotopes that are. Three naturally existing isotopes of hydrogen are tritium, deuterium, and protium. Atoms that have the same number of protons but different numbers of neutrons are known as isotopes. Web isotopes are substances that are composed of the same element but consist of different mass numbers and number of neutrons. If you understand how protons and electrons relate to one another,. Web explore the concepts of isotopes and atomic mass with this interactive simulation. Atoms of the same element with different mass numbers are called isotopes. Web isotopes are substances that are composed of the same element but consist of different mass numbers and number of neutrons. Web build an atom out of protons, neutrons, and electrons, and see how the. Web explore the concepts of isotopes and atomic mass with this interactive simulation. Web they discovered that heavy isotopes were left in the lunar soil, indicating that ion vaporization is the primary method of atmosphere generation on the moon. Learn how to use a mass spectrometer and compare different elements. What are some of the uses of radioactive isotopes? Web isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. The number of protons and the mass number of an atom define the type of atom. Atoms that have the same number of protons but different numbers of neutrons are known as isotopes. For example, carbon has six protons and is atomic number 6. They share the same atomic number and therefore the same number of. Protium is the most common isotope of hydrogen, and it has no neutrons. Plants find it easier to use the lighter isotopes (12 c) when they convert sunlight and carbon dioxide into food. Web build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! Web here are a few examples of isotopes: Additionally, n = a −z. Web high school students need concrete methods to get them engaged in learning about isotopes and atomic structure.
Isotopes of hydrogen. stock vector. Illustration of neutral 79319167
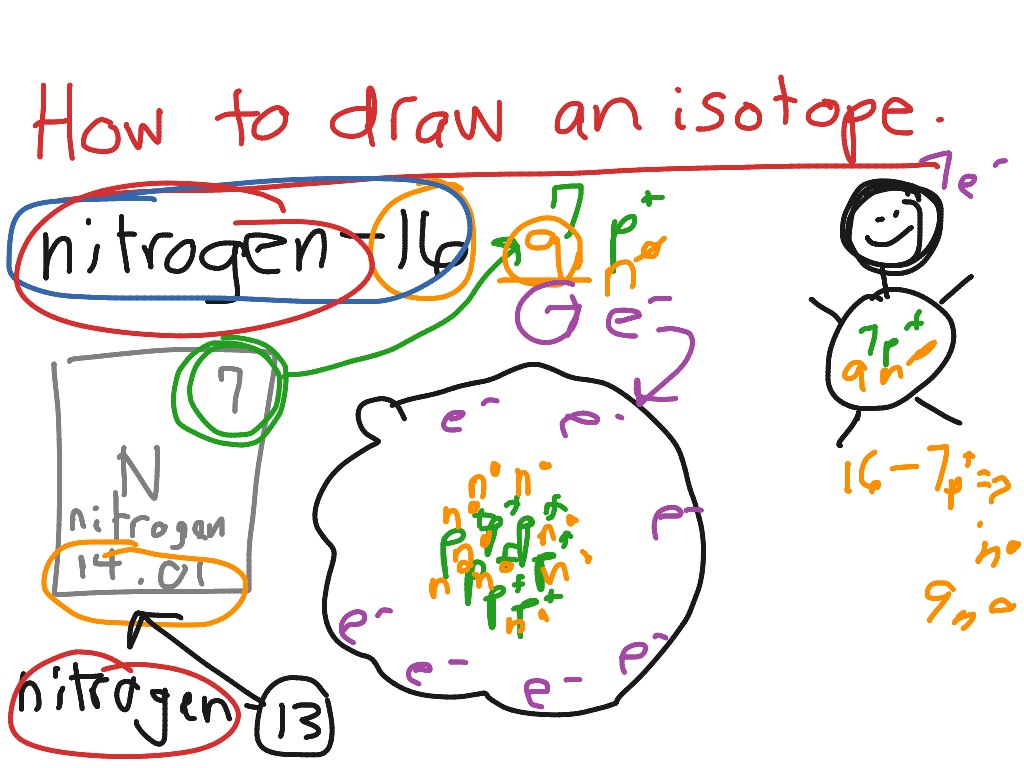
How to Draw an Isotope Science ShowMe
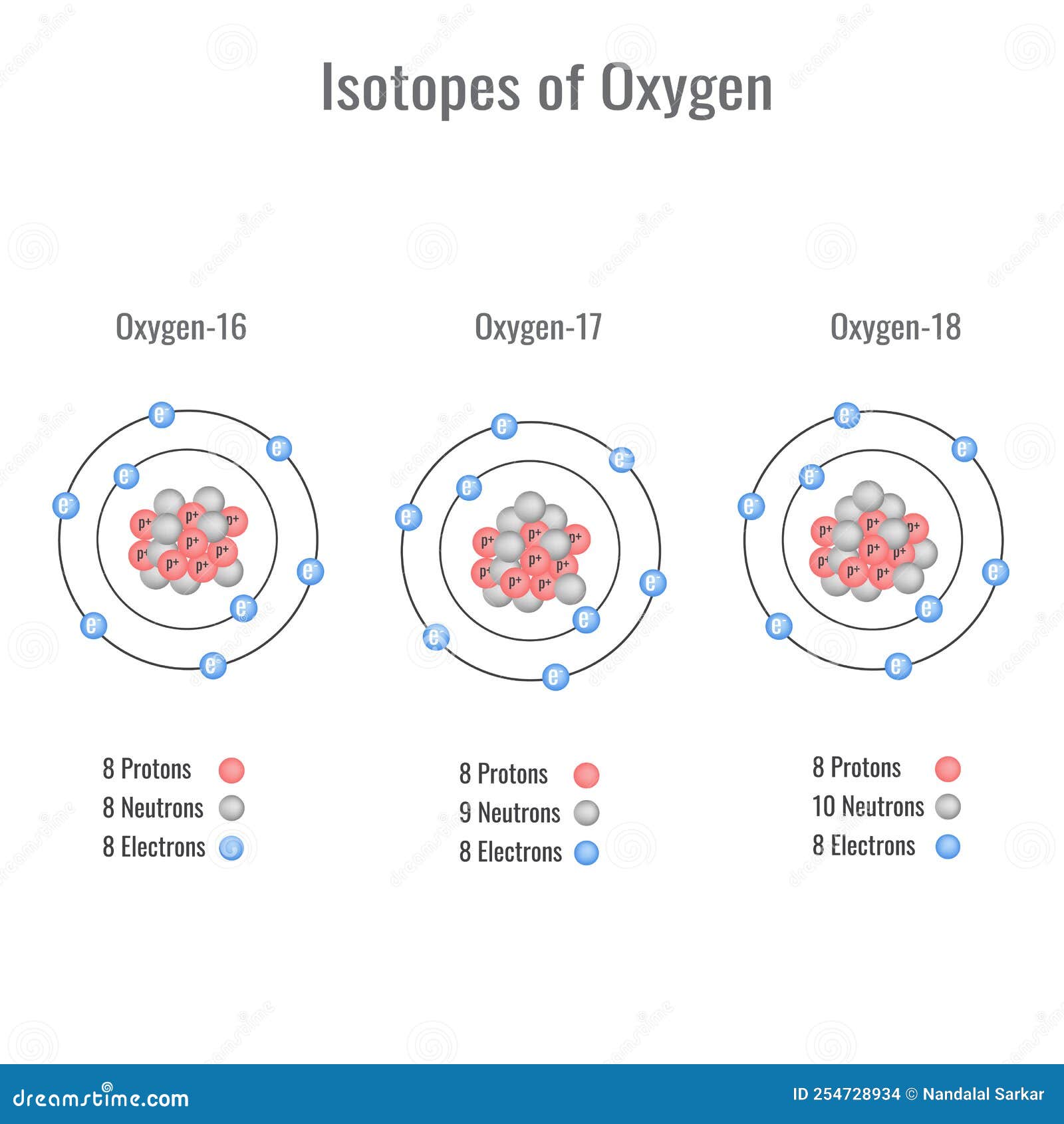
Isotopes of Oxygen Vector Illustration Stock Vector Illustration of
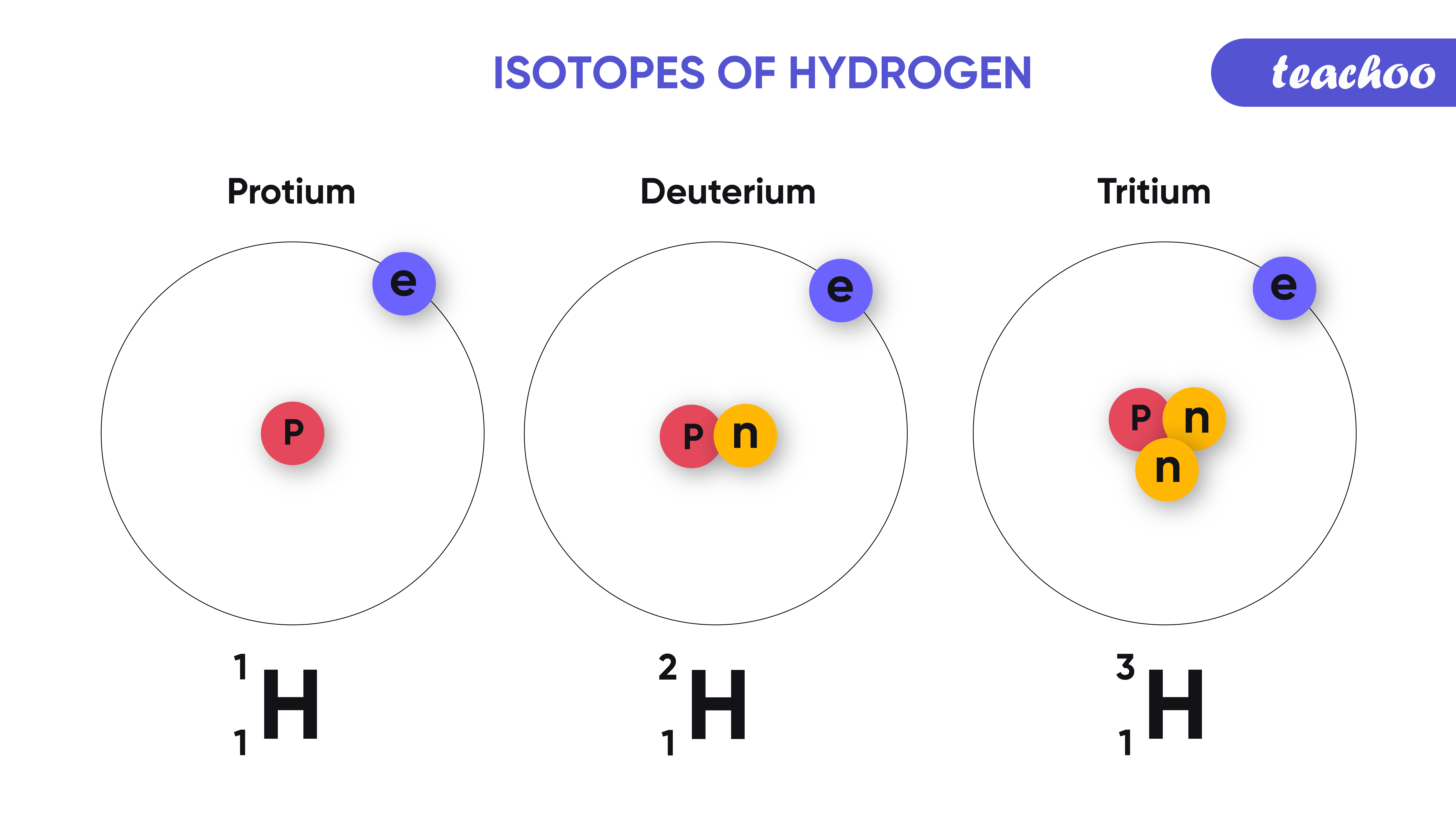
Isotopes and Isobars Definition, Uses and Difference Teachoo
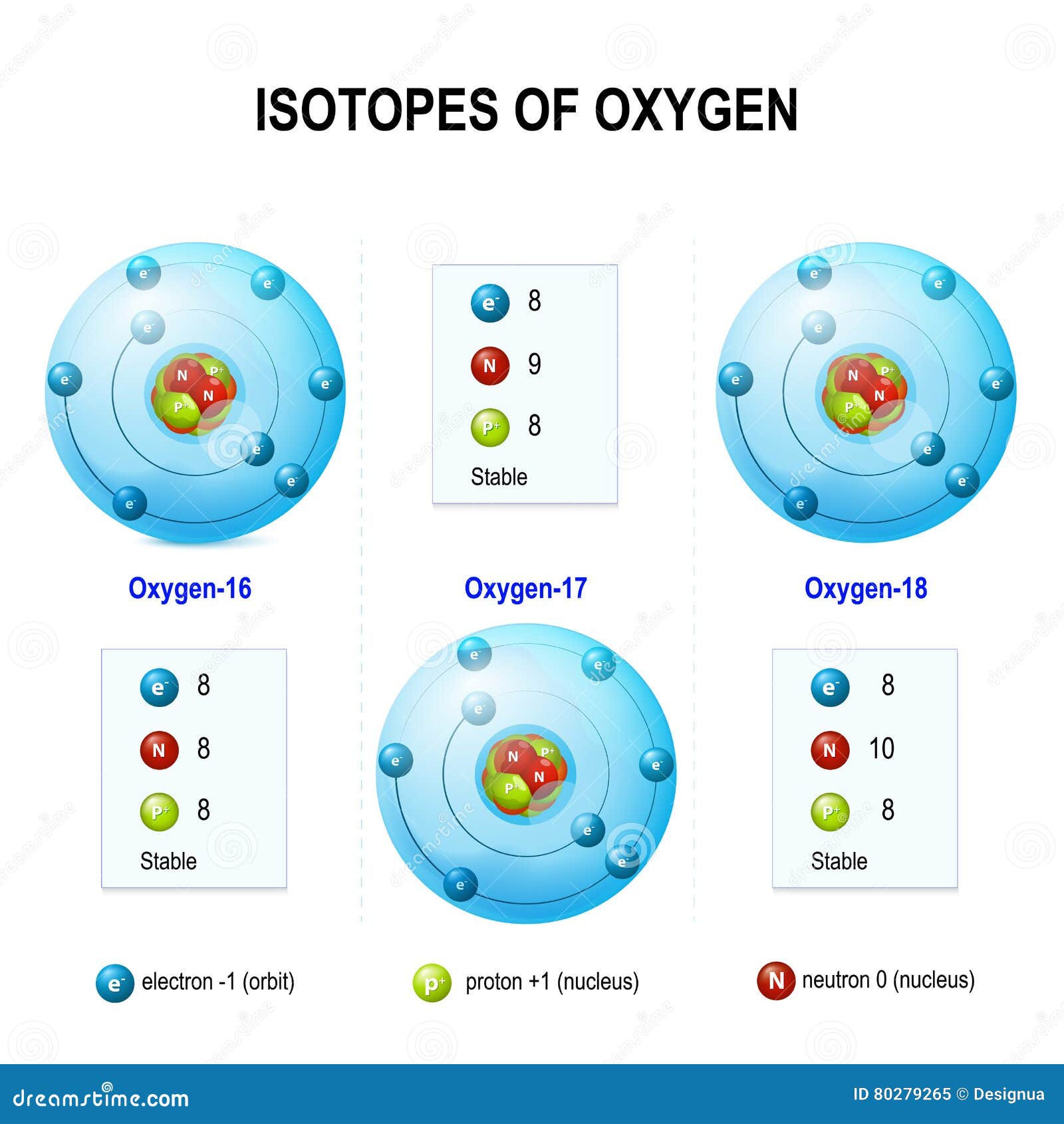
Isotopes of oxygen stock vector. Illustration of isotopes 80279265

Isotope Symbol Examples
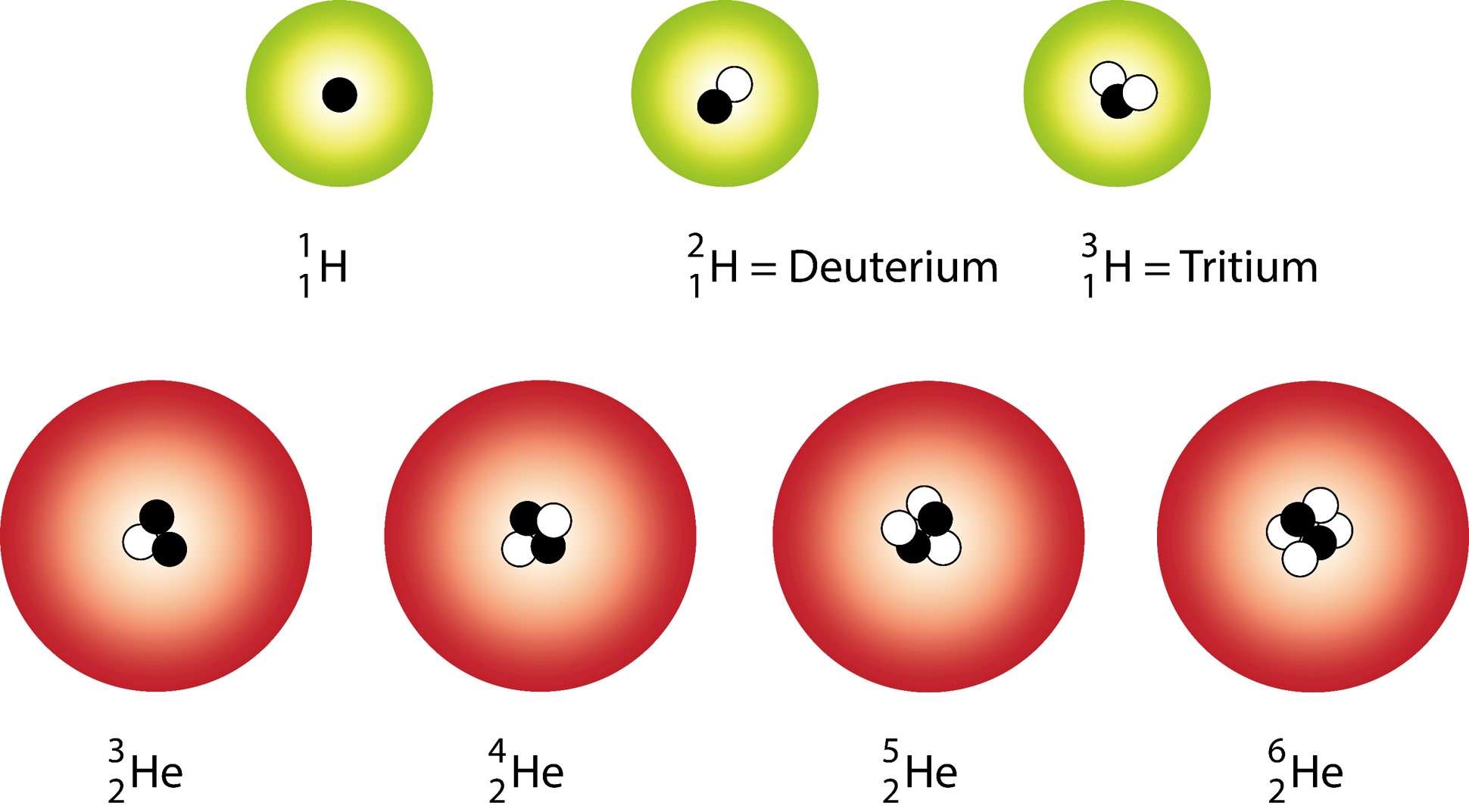
PreChemistry

Isotopes of carbon, illustration Stock Image C028/6464 Science
:max_bytes(150000):strip_icc()/Isotope-58dd6b415f9b5846830254ae.jpg)
Definition and Examples of Isotopes in Chemistry
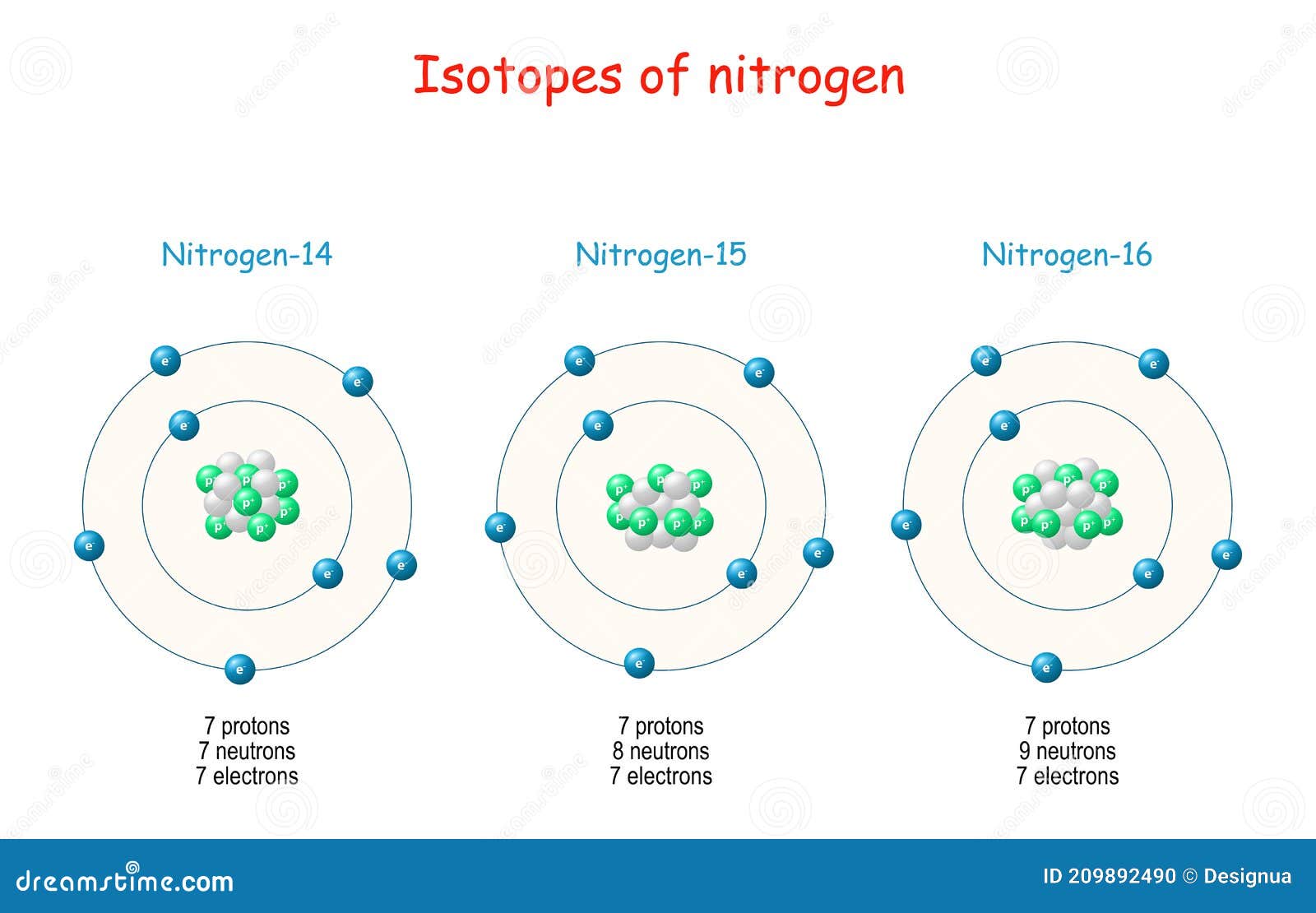
Nitrogen Isotopes. Structure of Atome Stock Vector Illustration of
Web Find The Mass Intensity Data Of Isotopes Here.
Three Naturally Existing Isotopes Of Hydrogen Are Tritium, Deuterium, And Protium.
Web The Specific Ratio Of Light To Heavy Isotopes That Remain In The Soil, For Both Potassium And Rubidium, Should Then Reveal The Main Process Contributing To The Lunar Atmosphere's Origins.
4 H To 7 H Are Nuclei Isotopes That Are Incorporated In The Laboratory.
Related Post: