Iqcp Template
Iqcp Template - Do the checklist requirements for iqcp apply to laboratories that are not subject to us regulations? Web describe the different components of an iqcp. Prepare laboratory records for inspection of an iqcp. Review new cap checklist requirements for iqcp. Use examples of iqcp to facilitate iqcp development. Identify additional resources for developing an iqcp. A new quality control (qc) option. Web a quality control plan (qcp) describes practices, procedures and resources needed by your laboratory to ensure the quality of a testing process. All disk diffusion and mic susceptibility testing with weekly qc will need iqcp. Labs performing gradient mic susceptibility testing with weekly qc will need iqcp. Why are we spending so much. You will evaluate your current quality activities and develop an iqcp worksheet which, when completed, can serve as your iqcp document. The qcp includes measures to assure the accuracy and reliability of test results, and that the quality of testing is adequate for patient care. Web discovering, deciphering and designing an individualized quality control. Review new cap checklist requirements for iqcp. Web discovering, deciphering and designing an individualized quality control plan (iqcp) iqcp provides a framework for customizing a quality control (qc) program for your test systems and your laboratory’s unique environment. Web the individualized quality control plan” (iqcp) is the clinical laboratory improvement amendments (clia) quality control (qc) procedure for an alternate qc. Web implement iqcp, when appropriate, and offer flexibility to design a qc plan that meets the needs of the laboratory. Web a quality control plan (qcp) describes practices, procedures and resources needed by your laboratory to ensure the quality of a testing process. Review new cap checklist requirements for iqcp. You will evaluate your current quality activities and develop an. Web discovering, deciphering and designing an individualized quality control plan (iqcp) iqcp provides a framework for customizing a quality control (qc) program for your test systems and your laboratory’s unique environment. Use examples of iqcp to facilitate iqcp development. Do the checklist requirements for iqcp apply to laboratories that are not subject to us regulations? A new quality control (qc). Why are we spending so much. The centers for medicare & medicaid services (cms) is implementing a new quality control option for laboratories based on risk management. Prepare laboratory records for inspection of an iqcp. Web discovering, deciphering and designing an individualized quality control plan (iqcp) iqcp provides a framework for customizing a quality control (qc) program for your test. You will evaluate your current quality activities and develop an iqcp worksheet which, when completed, can serve as your iqcp document. Web describe the different components of an iqcp. Web the individualized quality control plan” (iqcp) is the clinical laboratory improvement amendments (clia) quality control (qc) procedure for an alternate qc option allowed by 42cfr493.1250. Individualized quality control plan (iqcp):. By performing the steps in an iqcp, you will examine the potential sources of error in your Web a quality control plan (qcp) describes practices, procedures and resources needed by your laboratory to ensure the quality of a testing process. Web discovering, deciphering and designing an individualized quality control plan (iqcp) iqcp provides a framework for customizing a quality control. Identify additional resources for developing an iqcp. Why are we spending so much. Web a quality control plan (qcp) describes practices, procedures and resources needed by your laboratory to ensure the quality of a testing process. Web iqcp stands for individualized quality control plan and is the alternative clia quality control (qc) option that will provide for equivalent quality testing. Web the individualized quality control plan” (iqcp) is the clinical laboratory improvement amendments (clia) quality control (qc) procedure for an alternate qc option allowed by 42cfr493.1250. Web iqcp stands for individualized quality control plan and is the alternative clia quality control (qc) option that will provide for equivalent quality testing to meet the clia regulations for nonwaived tests. Web implement. Web implement iqcp, when appropriate, and offer flexibility to design a qc plan that meets the needs of the laboratory. Web iqcp stands for individualized quality control plan and is the alternative clia quality control (qc) option that will provide for equivalent quality testing to meet the clia regulations for nonwaived tests. Web describe the different components of an iqcp.. The qcp includes measures to assure the accuracy and reliability of test results, and that the quality of testing is adequate for patient care. Identify additional resources for developing an iqcp. A new quality control (qc) option. Web iqcp stands for individualized quality control plan and is the alternative clia quality control (qc) option that will provide for equivalent quality testing to meet the clia regulations for nonwaived tests. The centers for medicare & medicaid services (cms) is implementing a new quality control option for laboratories based on risk management. Review new cap checklist requirements for iqcp. Web implement iqcp, when appropriate, and offer flexibility to design a qc plan that meets the needs of the laboratory. Use examples of iqcp to facilitate iqcp development. Web discovering, deciphering and designing an individualized quality control plan (iqcp) iqcp provides a framework for customizing a quality control (qc) program for your test systems and your laboratory’s unique environment. Do the checklist requirements for iqcp apply to laboratories that are not subject to us regulations? Why are we spending so much. All disk diffusion and mic susceptibility testing with weekly qc will need iqcp. Labs performing gradient mic susceptibility testing with weekly qc will need iqcp. Prepare laboratory records for inspection of an iqcp. Individualized quality control plan (iqcp): Web describe the different components of an iqcp.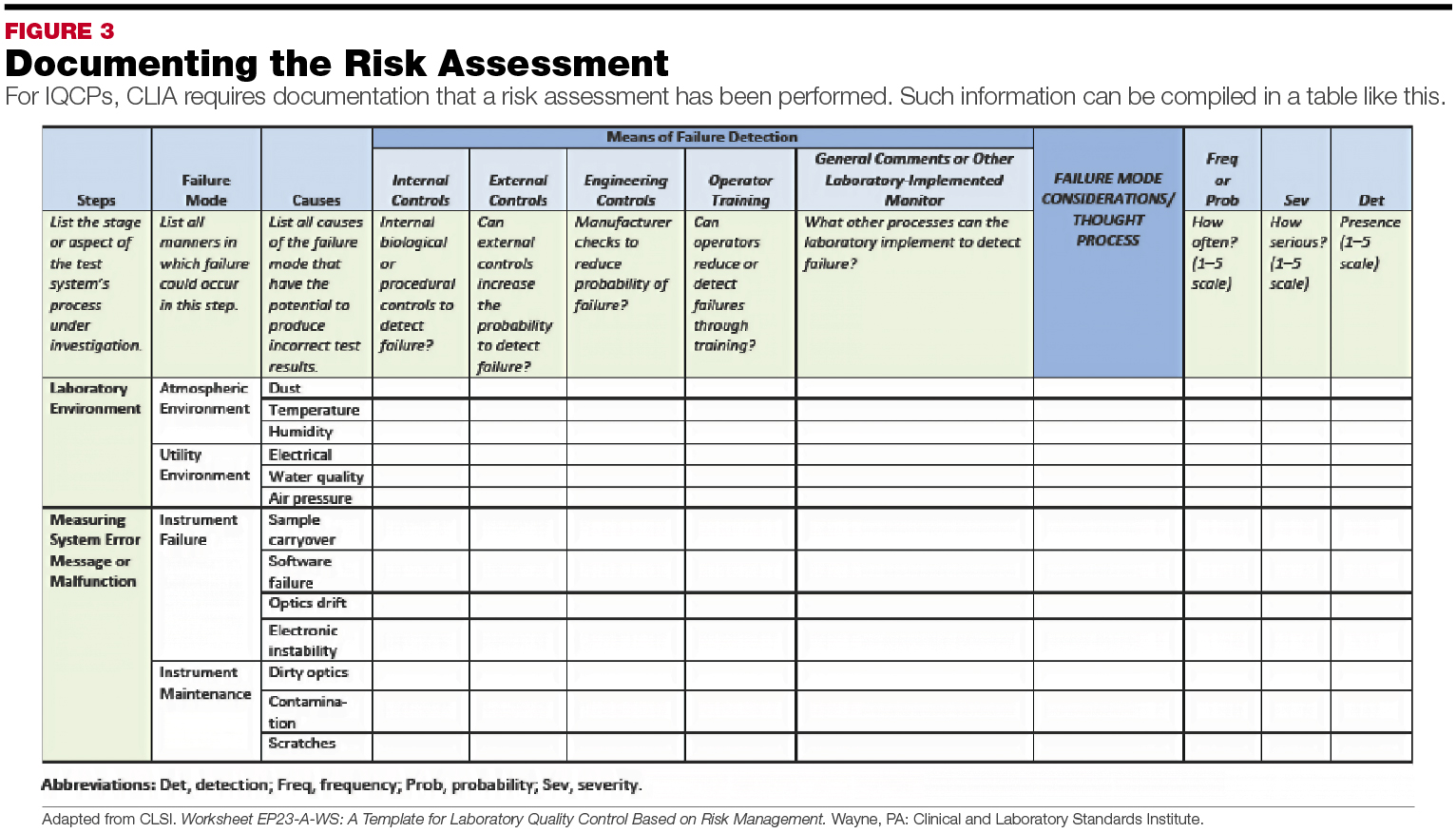
Develop Compliant IQCPs MarchApril 2015 MedicalLab Management Magazine

Individualized Quality Control Plan (IQCP)
Iqcp Template
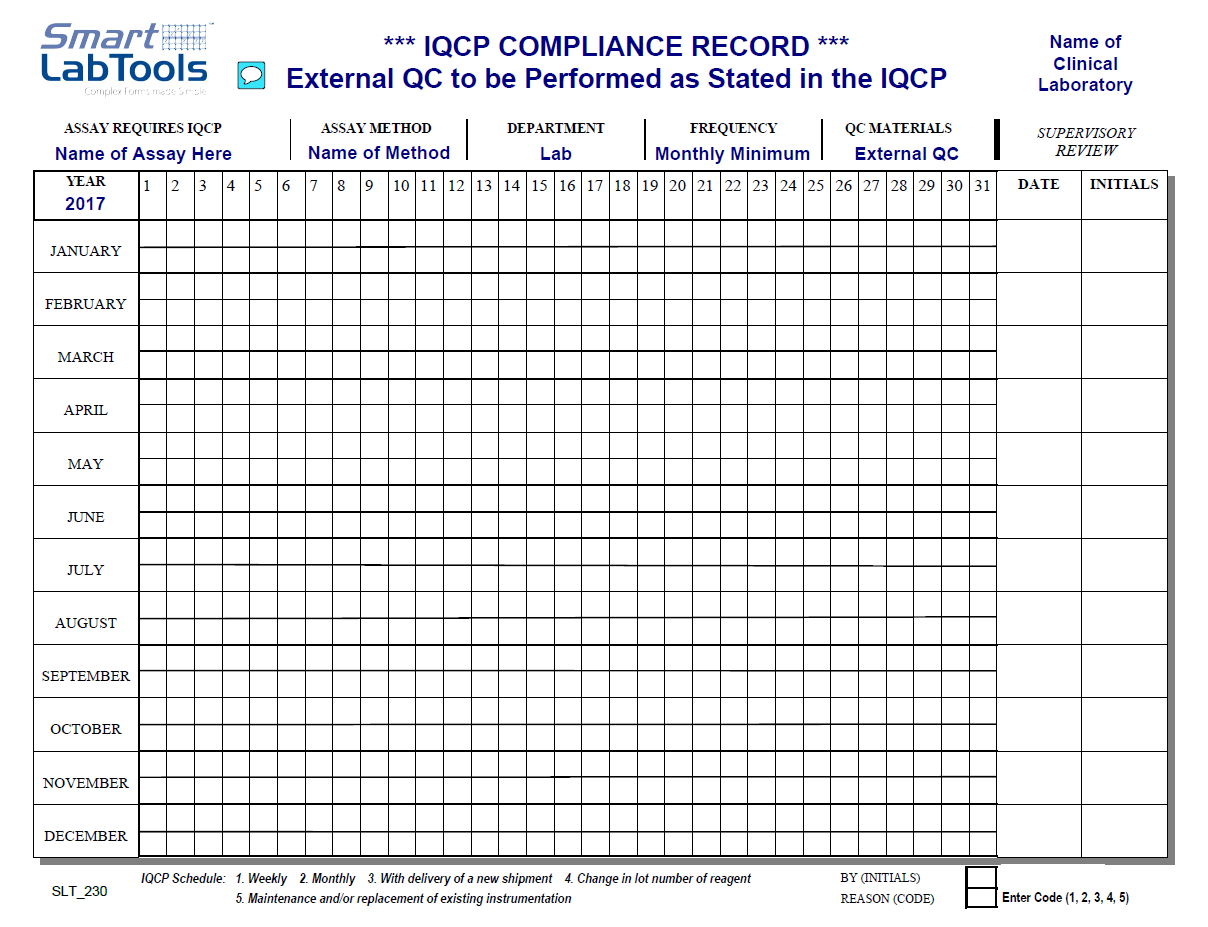
SmartLabTools SLT_IQCP Review Forms

Individualized Quality Control Plan (IQCP) Is It Value Doc
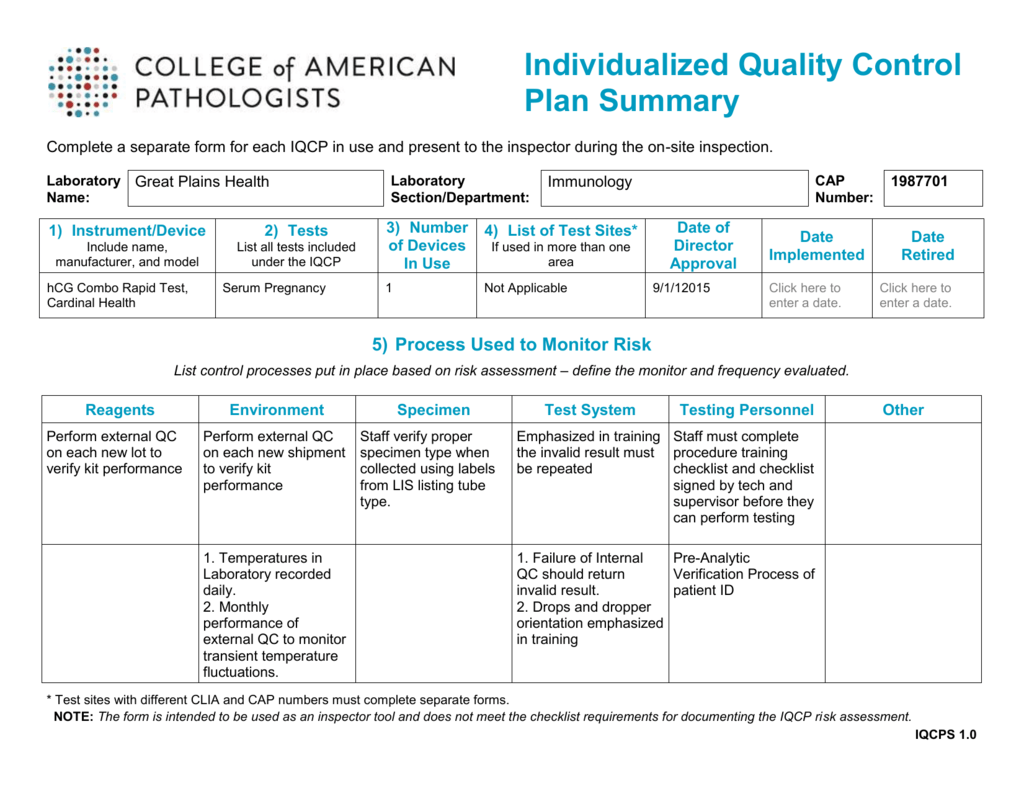
Individualized Quality Control Plan Summary
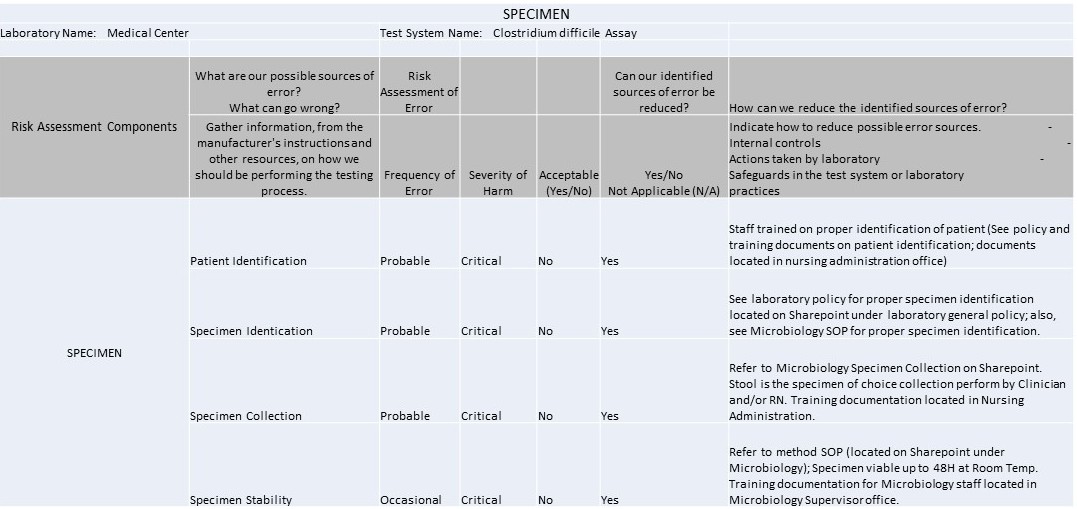
Example IQCP for C. Difficile Westgard

IQCP GUIDELINES and TEMPLATE FOR GETTING STARTED Doc Template pdfFiller
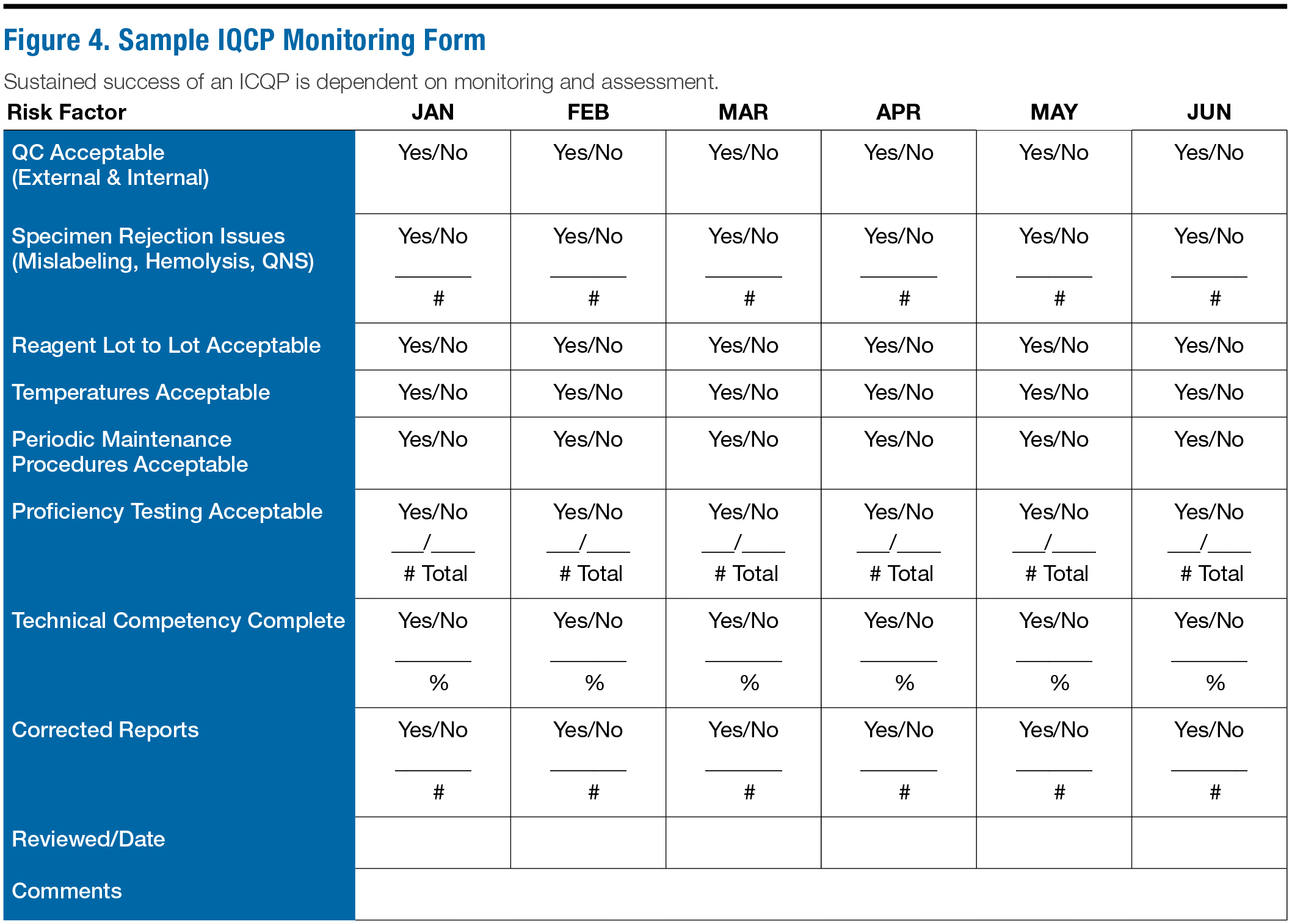
Iqcp Risk Assessment Template Flyer Template
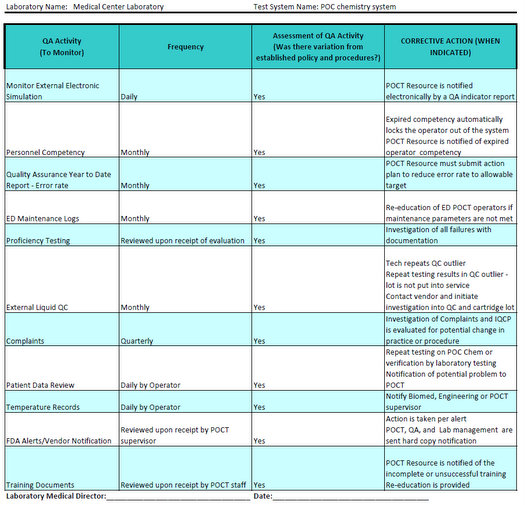
Example IQCP for POC chemistry system Westgard
Web The Individualized Quality Control Plan” (Iqcp) Is The Clinical Laboratory Improvement Amendments (Clia) Quality Control (Qc) Procedure For An Alternate Qc Option Allowed By 42Cfr493.1250.
You Will Evaluate Your Current Quality Activities And Develop An Iqcp Worksheet Which, When Completed, Can Serve As Your Iqcp Document.
By Performing The Steps In An Iqcp, You Will Examine The Potential Sources Of Error In Your
Web A Quality Control Plan (Qcp) Describes Practices, Procedures And Resources Needed By Your Laboratory To Ensure The Quality Of A Testing Process.
Related Post: