Iq Oq Pq Template
Iq Oq Pq Template - Use them right now to help with your qualification and validation projects. Installation qualification (iq), operational qualification (oq), and performance qualification (pq). Web performance qualification (pq) demonstrate the process will consistently produce acceptable product under normal operating conditions. Web what is iq, oq, pq? Web the objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the [insert system name and plant number] which will be located in the [insert area, packaging or manufacturing] at site [insert site name]. Web understanding iq, oq, and pq for medical device manufacturing processes. Web writing effective iq/oq/pq protocols is a must for following the regulations required by the fda for equipment, systems, and utilities to demonstrate suitability for the intended use and to operate according to their design and functional specifications. Web iq, oq, pq protocols are methods for demonstrating that equipment being used or installed will offer a high degree of quality assurance such that production processes will consistently manufacture products that meet quality requirements. Web write the objective of the protocol defining the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the equipment with location i.e., packaging or manufacturing, and the facility. Web these iq oq pq template contains over seventeen fully detailed qualification test scripts along with the methodology for twenty more compliance tests and inspections. Use them right now to help with your qualification and validation projects. The goal of process validation is to produce a stable medical device manufacturing process that offers consistent performance. Web the objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the [insert system name and plant number] which. Installation qualification (iq), operational qualification (oq), and performance qualification (pq). Use them right now to help with your qualification and validation projects. Web writing effective iq/oq/pq protocols is a must for following the regulations required by the fda for equipment, systems, and utilities to demonstrate suitability for the intended use and to operate according to their design and functional specifications.. Web what is iq, oq, pq? These are the abbreviations we use in the medical device industry for the three steps of process validation: Web write the objective of the protocol defining the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the equipment with location i.e., packaging or manufacturing, and the facility. Installation qualification (iq) for. These are the abbreviations we use in the medical device industry for the three steps of process validation: Web performance qualification (pq) demonstrate the process will consistently produce acceptable product under normal operating conditions. Web understanding iq, oq, and pq for medical device manufacturing processes. Web these iq oq pq template contains over seventeen fully detailed qualification test scripts along. Web the objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the [insert system name and plant number] which will be located in the [insert area, packaging or manufacturing] at site [insert site name]. Web performance qualification (pq) demonstrate the process will consistently produce acceptable product under normal operating. Use them right now to help with your qualification and validation projects. These are the abbreviations we use in the medical device industry for the three steps of process validation: Web what is iq, oq, pq? Installation qualification (iq), operational qualification (oq), and performance qualification (pq). Web the objective of this protocol is to define the installation qualification (iq) and. Use them right now to help with your qualification and validation projects. Web iq, oq, pq protocols are methods for demonstrating that equipment being used or installed will offer a high degree of quality assurance such that production processes will consistently manufacture products that meet quality requirements. Things to consider… • approved procedures and limits. Web writing effective iq/oq/pq protocols. Web performance qualification (pq) demonstrate the process will consistently produce acceptable product under normal operating conditions. Web understanding iq, oq, and pq for medical device manufacturing processes. Web what is iq, oq, pq? Installation qualification (iq) for hardware verifies that the physical equipment and ancillary systems are installed correctly and in accordance with manufacturer specifications and regulatory requirements. The goal. Web these iq oq pq template contains over seventeen fully detailed qualification test scripts along with the methodology for twenty more compliance tests and inspections. Use them right now to help with your qualification and validation projects. Installation qualification (iq), operational qualification (oq), and performance qualification (pq). Things to consider… • approved procedures and limits. Web what is iq, oq,. Web these iq oq pq template contains over seventeen fully detailed qualification test scripts along with the methodology for twenty more compliance tests and inspections. Web what is iq, oq, pq? Web iq, oq, pq protocols are methods for demonstrating that equipment being used or installed will offer a high degree of quality assurance such that production processes will consistently. Web what is iq, oq, pq? Web understanding iq, oq, and pq for medical device manufacturing processes. Web writing effective iq/oq/pq protocols is a must for following the regulations required by the fda for equipment, systems, and utilities to demonstrate suitability for the intended use and to operate according to their design and functional specifications. Web iq, oq, pq protocols are methods for demonstrating that equipment being used or installed will offer a high degree of quality assurance such that production processes will consistently manufacture products that meet quality requirements. Installation qualification (iq) for hardware verifies that the physical equipment and ancillary systems are installed correctly and in accordance with manufacturer specifications and regulatory requirements. Web what is iq, oq, pq? Web write the objective of the protocol defining the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the equipment with location i.e., packaging or manufacturing, and the facility. Web these iq oq pq template contains over seventeen fully detailed qualification test scripts along with the methodology for twenty more compliance tests and inspections. These are the abbreviations we use in the medical device industry for the three steps of process validation: Things to consider… • approved procedures and limits. Installation qualification (iq), operational qualification (oq), and performance qualification (pq). The goal of process validation is to produce a stable medical device manufacturing process that offers consistent performance.
Iq Oq Pq Templates
Iq Oq Pq Full Form
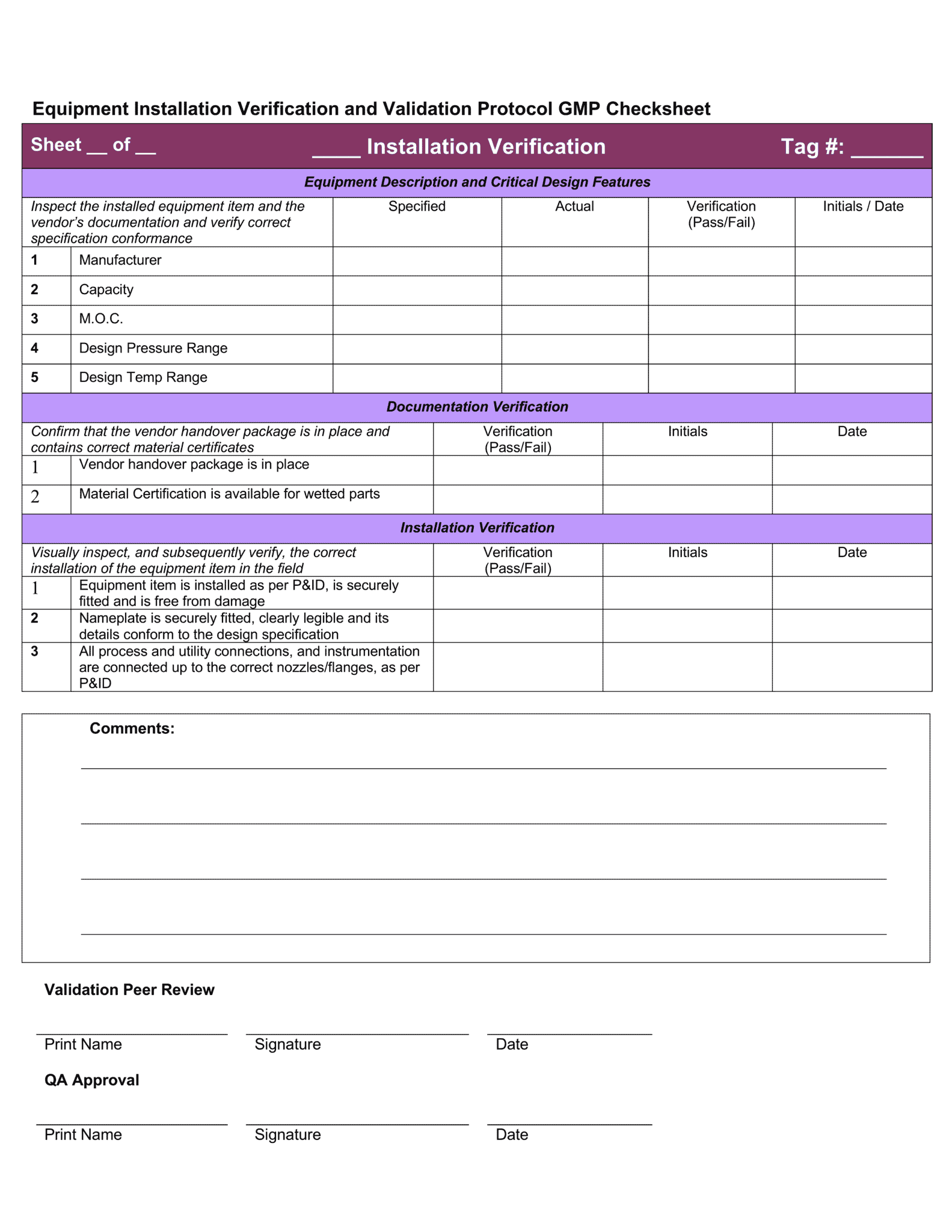
IQ OQ PQ Templates Download 4 Professional Templates

Six Sigma Validation Process IQ Installation Qualification OQ
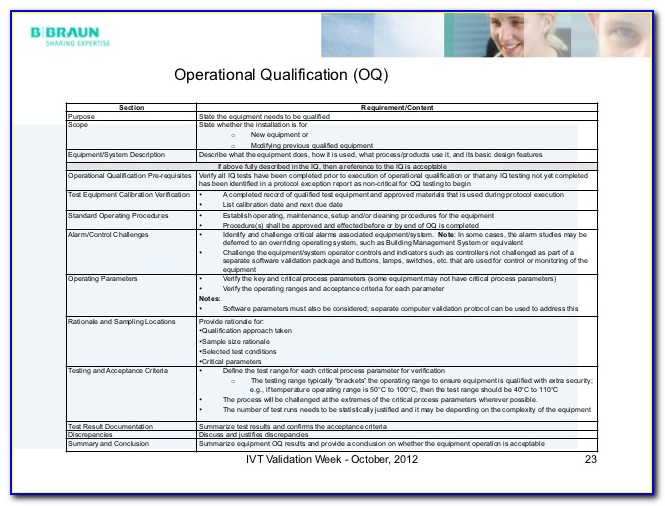
Iq Oq Pq Templates
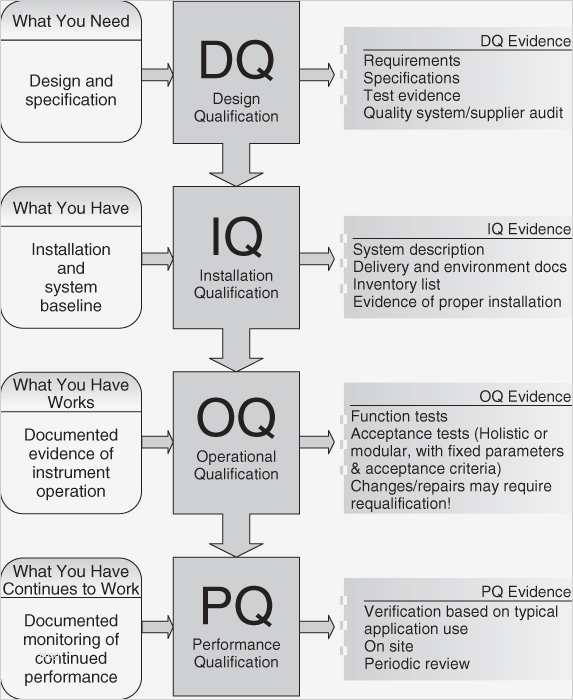
Iq Oq Pq Vorlage Gut Iq Oq Pq Validation Templates Template Design

Iq Oq Pq Templates
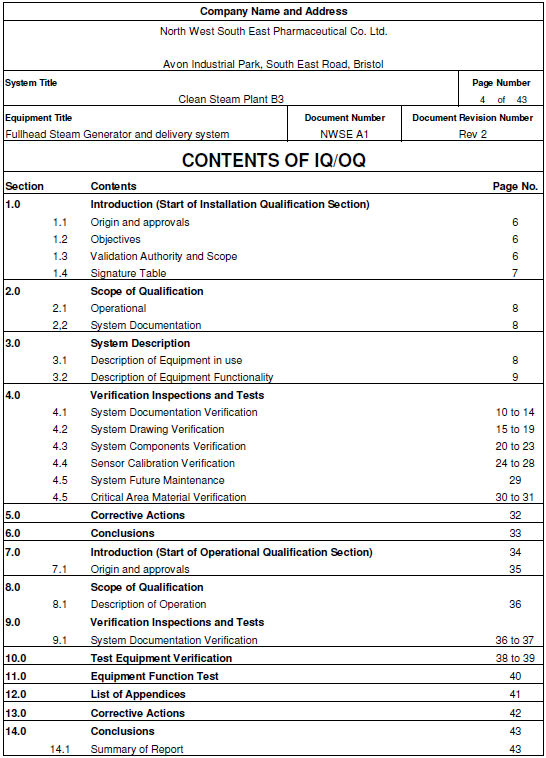
IQ, OQ, PQ Steam Quality Qualification Documentation
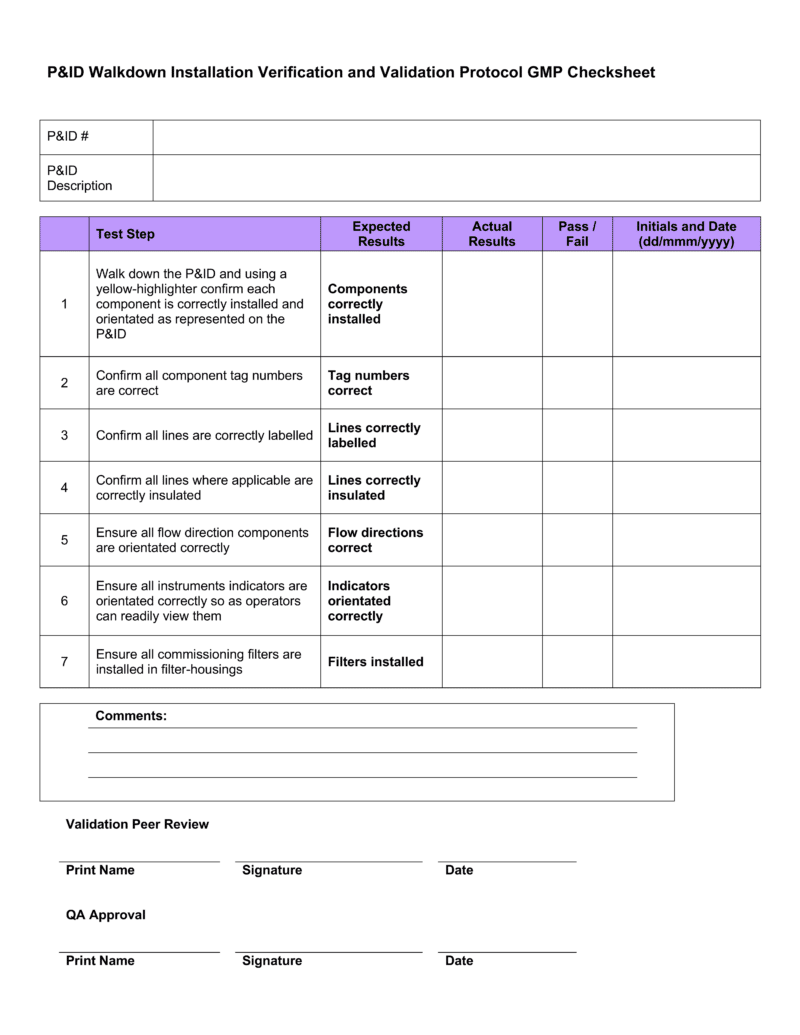
Download 4 professional IQ OQ PQ templates GetReskilled
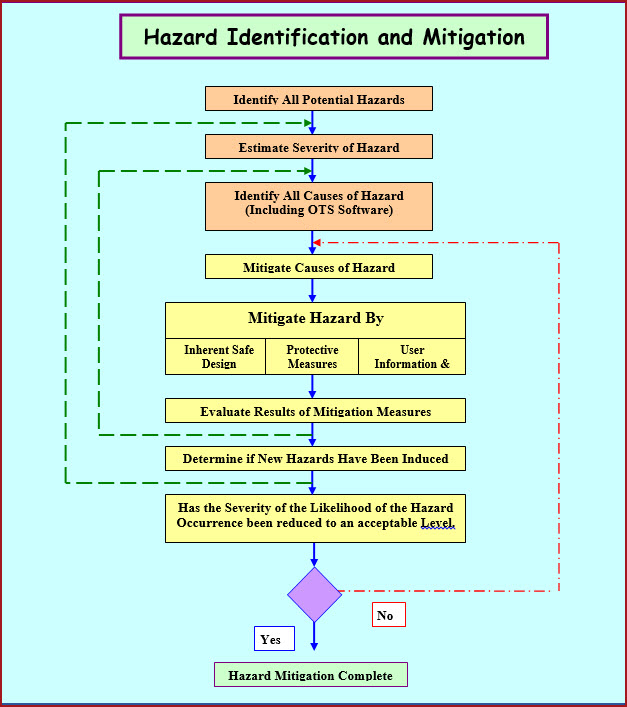
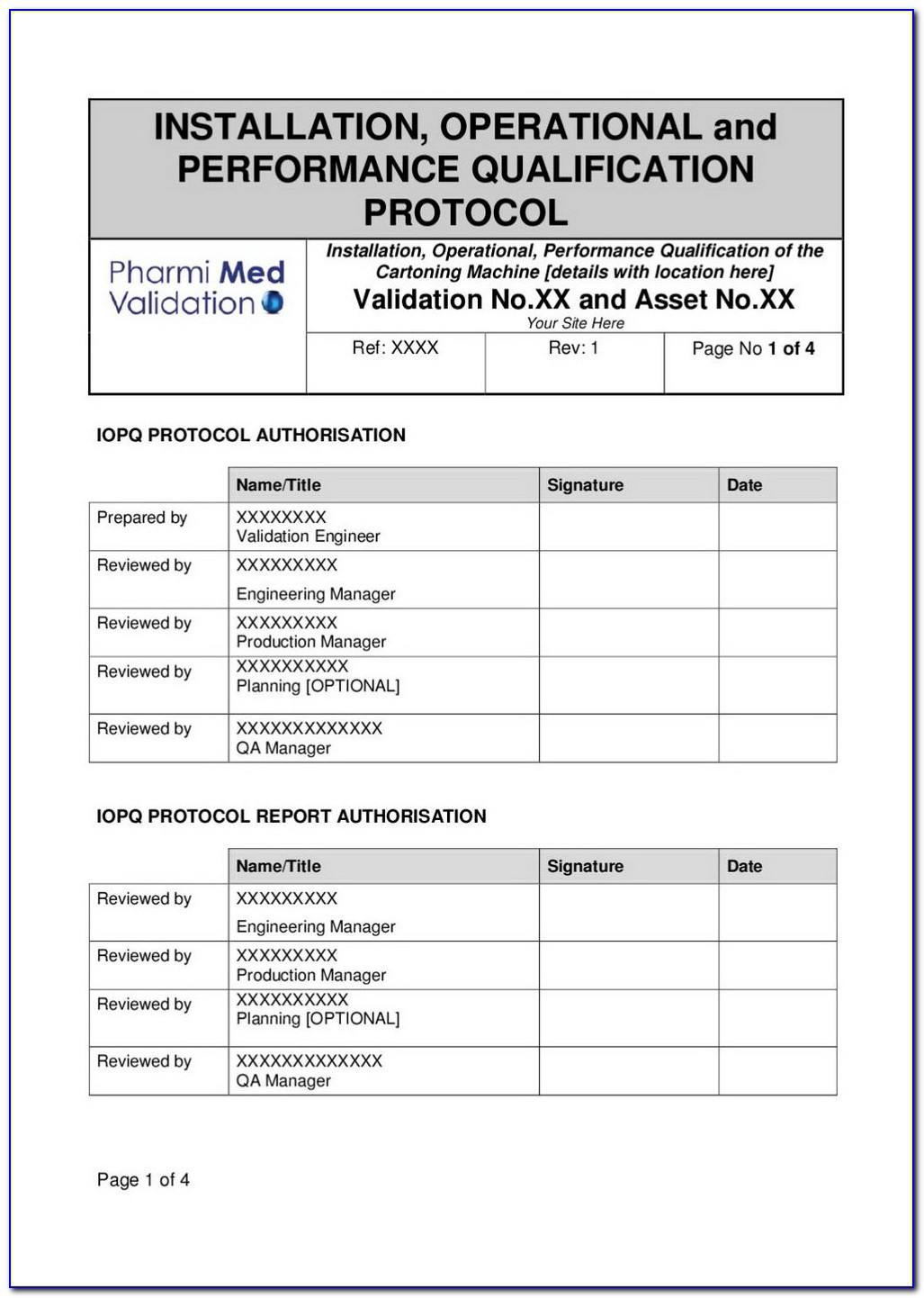
How to produce a superb combined IQ OQ PQ Protocol.
Web Performance Qualification (Pq) Demonstrate The Process Will Consistently Produce Acceptable Product Under Normal Operating Conditions.
Web The Objective Of This Protocol Is To Define The Installation Qualification (Iq) And Operational Qualification (Oq) Requirements And Acceptance Criteria For The [Insert System Name And Plant Number] Which Will Be Located In The [Insert Area, Packaging Or Manufacturing] At Site [Insert Site Name].
Use Them Right Now To Help With Your Qualification And Validation Projects.
Related Post: