Ionization Energy Chart Periodic Table
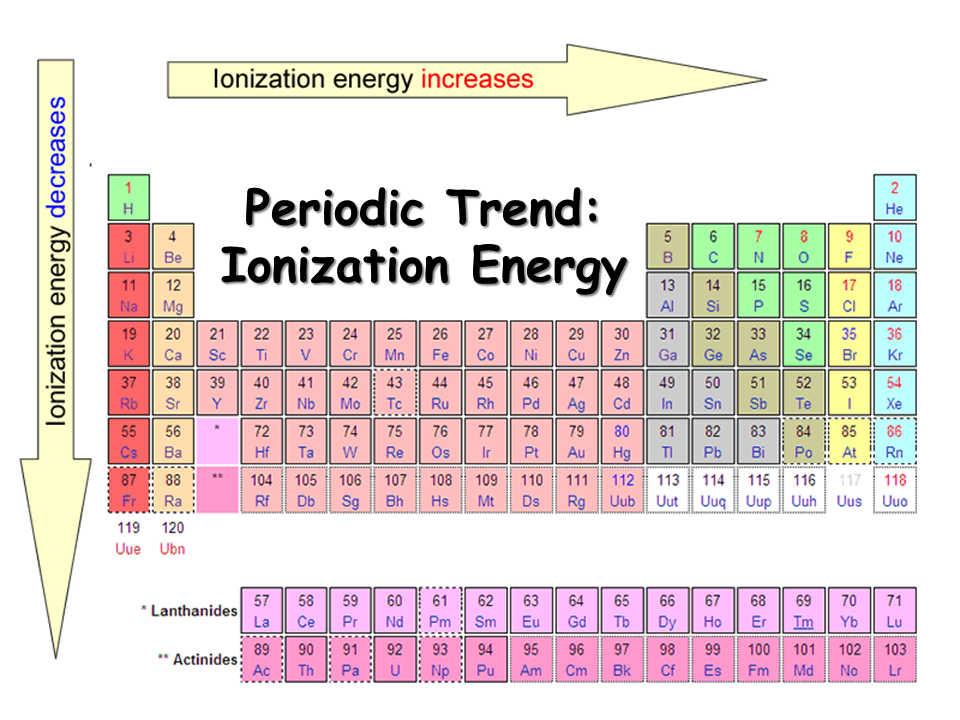
Ionization Energy Chart Periodic Table - Web an element's first ionization energy is the energy required to remove the outermost, or least bound, electron from a neutral atom of the element. Web the periodic table of the elements (with ionization energies) element name. Web what is ionization energy? Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. On the periodic table, first ionization energy generally increases as you move left to right across a period. The table lists only the first ie in ev units. This version of the periodic table shows the first ionization energy of (ie: Web for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron from the +1 ion, the column marked 3 is the third ionization energy to remove a third electron from the +2 ion, and so on. The ionization energy differs for each atom. Web ionization energy chart of all the elements is given below. Web this list contains the 118 elements of chemistry. You can also get the hd printable periodic table with ionization energy, from this article only. Web table \(\pageindex{1}\) gives the ionization energies for the period three elements. Web what trends do you see on the periodic table for ionization energy and what causes these trends? Web for each atom, the. Web this list contains the 118 elements of chemistry. The unity for ionization energy is ev. The table lists only the first ie in ev units. Web the 1st ionization energy of the element m is a measure of the energy required to remove one electron from one mole of the gaseous atoms m. The energy required to remove the. The first chemical element is cesium and the last one is helium. Web ionization energy is the energy needed to remove an electron from an atom or ion. Web the periodic table of the elements (with ionization energies) element name. First ionization energy, second ionization energy as well as third ionization energy of the elements are given in this chart.. Web what is ionization energy? The energy required to remove the outermost electron from an atom or a positive ion in its ground level. Notice how the second ionization energy for sodium, third ionization energy for magnesium, fourth ionization energy for aluminum, etc. The unity for ionization energy is ev. On the periodic table, first ionization energy generally increases as. The stronger an electron is bound to an atom the more ionization energy it requires, therefore these two are directly proportional. Web ionization energy chart of all the elements is given below. Web periodic behavior is most evident for ionization energy (i), the energy required to remove an electron from a gaseous atom. Web the periodic table of the elements. Now, what about trends up and down the periodic table? Web an element's first ionization energy is the energy required to remove the outermost, or least bound, electron from a neutral atom of the element. Web what is ionization energy? You can also get the hd printable periodic table with ionization energy, from this article only. 1), in kj/mol, of. The measurement is performed in the gas phase on single atoms. To convert to kj/mol, multiply by 96.4869. The first molar ionization energy applies to the neutral atoms. Now, what about trends up and down the periodic table? First ionization energy, second ionization energy as well as third ionization energy of the elements are given in this chart. The first chemical element is cesium and the last one is helium. Nist reference table on ground states and ionization energies for the neutral atoms. Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. How would you write a chemical equation representing. Web ionization energy is the amount of energy needed. The table lists only the first ie in ev units. Web the ionization energy is a measure of the energy required to remove one electron from one mole of gaseous atoms or ions. Web table \(\pageindex{1}\) gives the ionization energies for the period three elements. 1), in kj/mol, of selected elements. Web periodic behavior is most evident for ionization energy. The energy required to remove the outermost electron from an atom or a positive ion in its ground level. Web these tables list values of molar ionization energies, measured in kj⋅mol −1. Web the ionization energy of atoms, denoted e i, is measured by finding the minimal energy of light quanta or electrons accelerated to a known energy that will. The ionization energy differs for each atom. Web the ionization energy is a measure of the energy required to remove one electron from one mole of gaseous atoms or ions. The stronger an electron is bound to an atom the more ionization energy it requires, therefore these two are directly proportional. Image showing periodicity of the chemical elements for ionization energy: On the periodic table, first ionization energy generally decreases as you move down a group. The table lists only the first ie in ev units. There are trends that match the structure of the periodic table. Web an element's first ionization energy is the energy required to remove the outermost, or least bound, electron from a neutral atom of the element. Nist reference table on ground states and ionization energies for the neutral atoms. Notice how the second ionization energy for sodium, third ionization energy for magnesium, fourth ionization energy for aluminum, etc. Web what trends do you see on the periodic table for ionization energy and what causes these trends? Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. The first molar ionization energy applies to the neutral atoms. Web what is ionization energy? The energy required to remove the outermost electron from an atom or a positive ion in its ground level. Now, what about trends up and down the periodic table?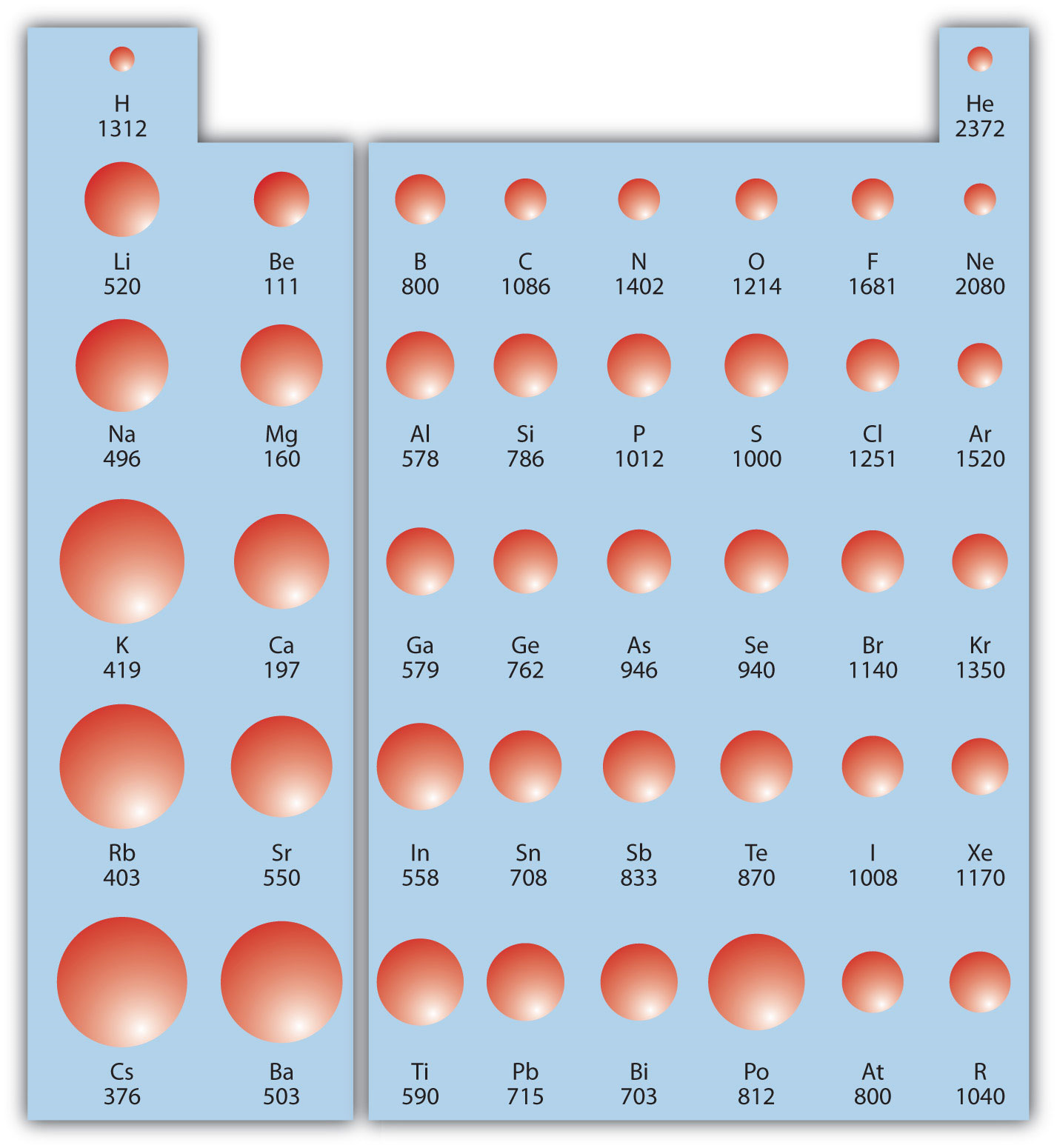
9.9 Periodic Trends Atomic Size, Ionization Energy, and Metallic

Periodic Trends in Ionization Energy Chemistry Socratic
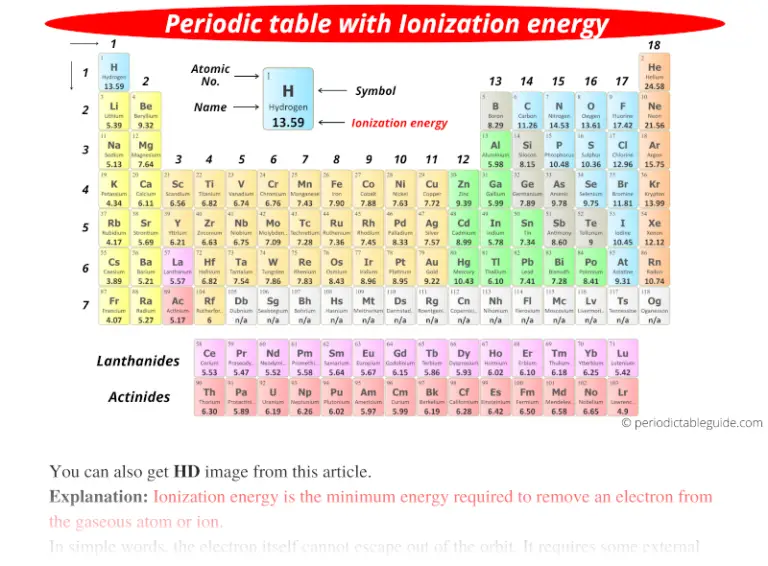
Periodic table with Ionization Energy Values (Labeled Image)

Periodic Table Ionization Energy Labeled

The Parts of the Periodic Table
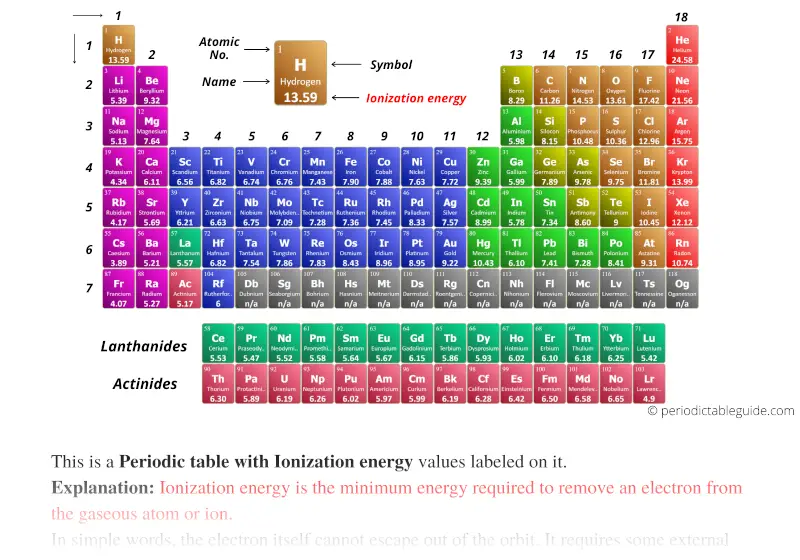
Periodic table with Ionization Energy Values (Labeled Image)

Periodic Properties of the Elements Chemwiki
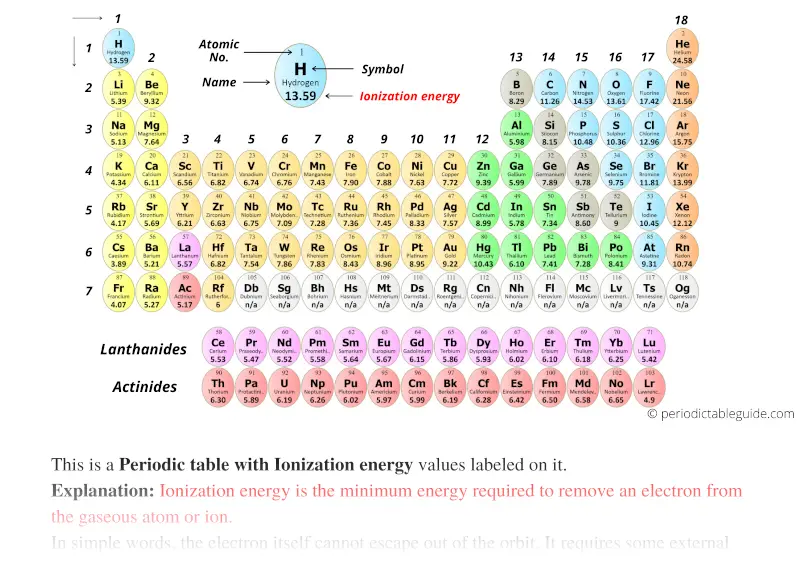
Periodic table with Ionization Energy Values (Labeled Image)

Ionization Energy Chart Periodic Table
Ionization Enthalpy NEET Lab
The First Chemical Element Is Cesium And The Last One Is Helium.
First Ionization Energy, Second Ionization Energy As Well As Third Ionization Energy Of The Elements Are Given In This Chart.
Web Ionization Energy Is The Energy Needed To Remove An Electron From An Atom Or Ion.
This Is The Energy Per Mole Necessary To Remove Electrons From Gaseous Atoms Or Atomic Ions.
Related Post: