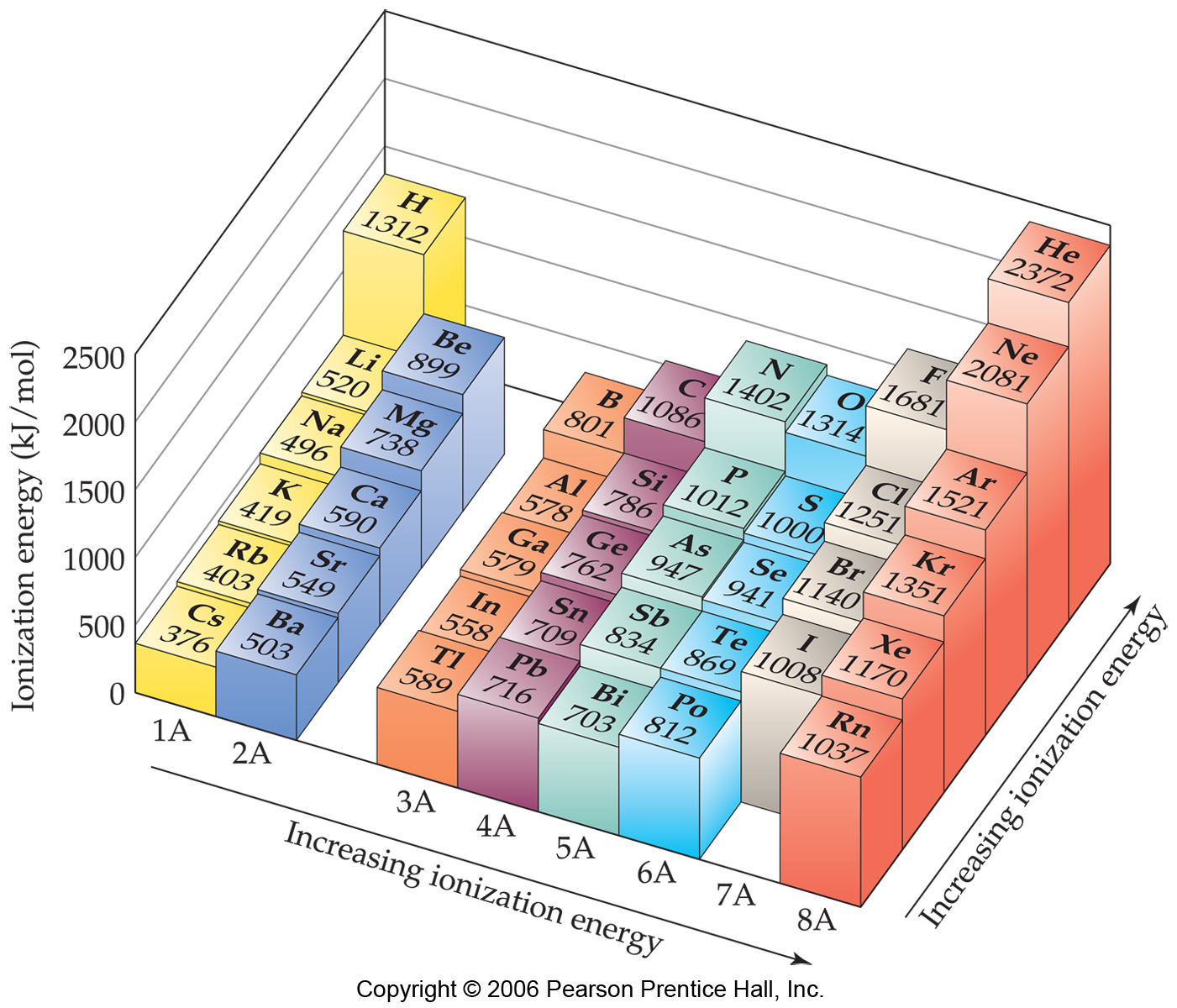
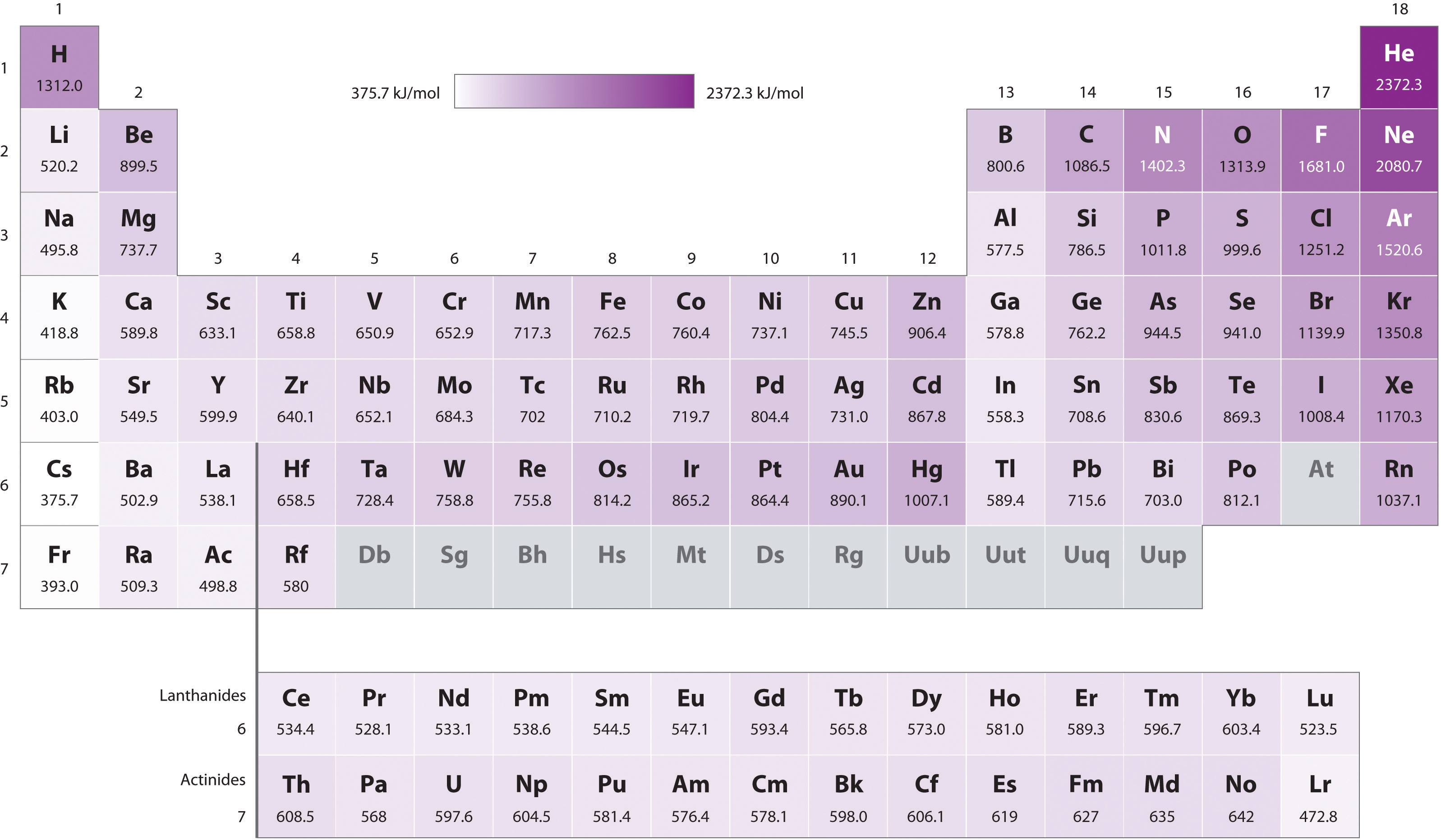
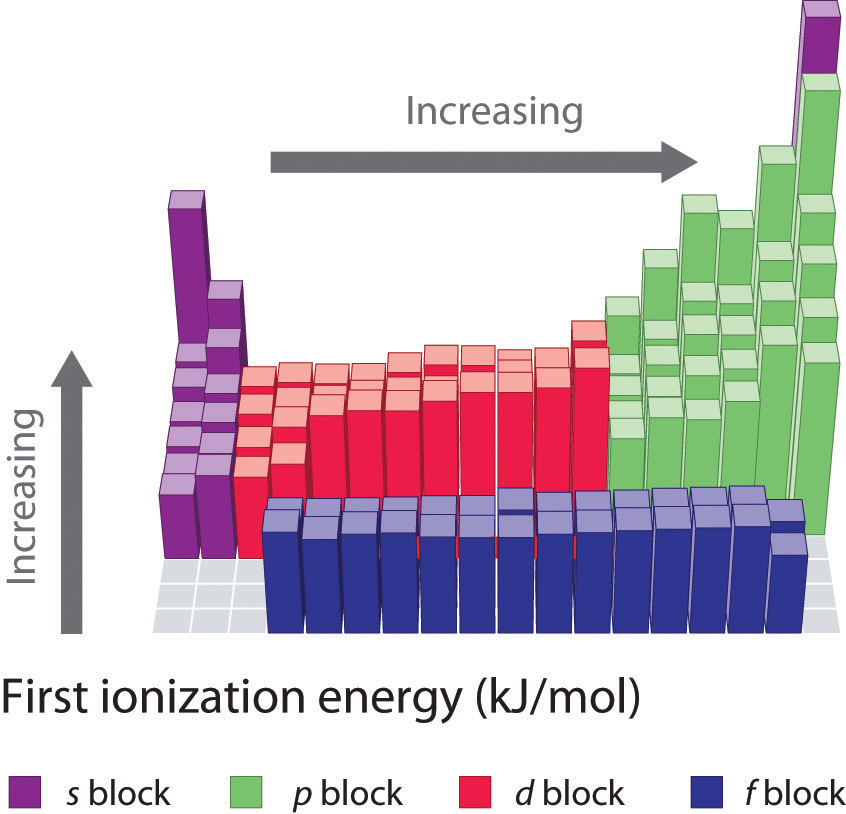
Ionization Chart
Ionization Chart - Web these tables list values of molar ionization energies, measured in kj⋅mol −1. Also, learn first & second ionization energies. Web this list contains the 118 elements of chemistry. Web ionization energy chart of all the elements is given below. The first chemical element is cesium and the last one is helium. The tabular chart on the right is arranged by ionization energy. I i is therefore the energy required for the reaction. Web for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron from the +1 ion, the column marked 3 is the third ionization energy to remove a third electron from the +2 ion, and so on. The unity for ionization energy is ev. Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. I i is therefore the energy required for the reaction. Web for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron from the +1 ion, the column marked 3 is the third ionization energy to remove a third electron. Web what is ionization energy. The first chemical element is cesium and the last one is helium. Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. The unity for ionization energy is ev. The first molar ionization energy applies to the neutral atoms. Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. Web these tables list values of molar ionization energies, measured in kj⋅mol −1. Web this list contains the 118 elements of chemistry. The first chemical element is cesium and the last one is helium. The unity for ionization energy is ev. The tabular chart on the right is arranged by ionization energy. The first chemical element is cesium and the last one is helium. Web what is ionization energy. Also, learn first & second ionization energies. Web ionization energy chart of all the elements is given below. Web these tables list values of molar ionization energies, measured in kj⋅mol −1. E(g) → e+(g) +e− energy required=i (7.4.1) (7.4.1) e ( g) → e ( g) + + e − energy required=i. This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. Also, learn first & second ionization energies. Web what is. The first molar ionization energy applies to the neutral atoms. Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. Web this list contains the 118 elements of chemistry. Web these tables list values of molar ionization energies, measured in kj⋅mol −1. Web chemists define the ionization energy ( i i) of. Web chemists define the ionization energy ( i i) of an element as the amount of energy needed to remove an electron from the gaseous atom e e in its ground state. Learn its chemical equation, values, trends across a period & down a group, & exception. The first molar ionization energy applies to the neutral atoms. The tabular chart. The first molar ionization energy applies to the neutral atoms. The unity for ionization energy is ev. For chemistry students and teachers: Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. The tabular chart on the right is arranged by ionization energy. The first molar ionization energy applies to the neutral atoms. First ionization energy, second ionization energy as well as third ionization energy of the elements are given in this chart. Web ionization energy chart of all the elements is given below. I i is therefore the energy required for the reaction. Learn its chemical equation, values, trends across a period. The first chemical element is cesium and the last one is helium. Web ionization energy chart of all the elements is given below. For chemistry students and teachers: The unity for ionization energy is ev. Web for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second. Web these tables list values of molar ionization energies, measured in kj⋅mol −1. The unity for ionization energy is ev. Web ionization energy chart of all the elements is given below. Web for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron from the +1 ion, the column marked 3 is the third ionization energy to remove a third electron from the +2 ion, and so on. Web chemists define the ionization energy ( i i) of an element as the amount of energy needed to remove an electron from the gaseous atom e e in its ground state. Web what is ionization energy. The tabular chart on the right is arranged by ionization energy. E(g) → e+(g) +e− energy required=i (7.4.1) (7.4.1) e ( g) → e ( g) + + e − energy required=i. Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. Learn its chemical equation, values, trends across a period & down a group, & exception. Web this list contains the 118 elements of chemistry. I i is therefore the energy required for the reaction. The first chemical element is cesium and the last one is helium.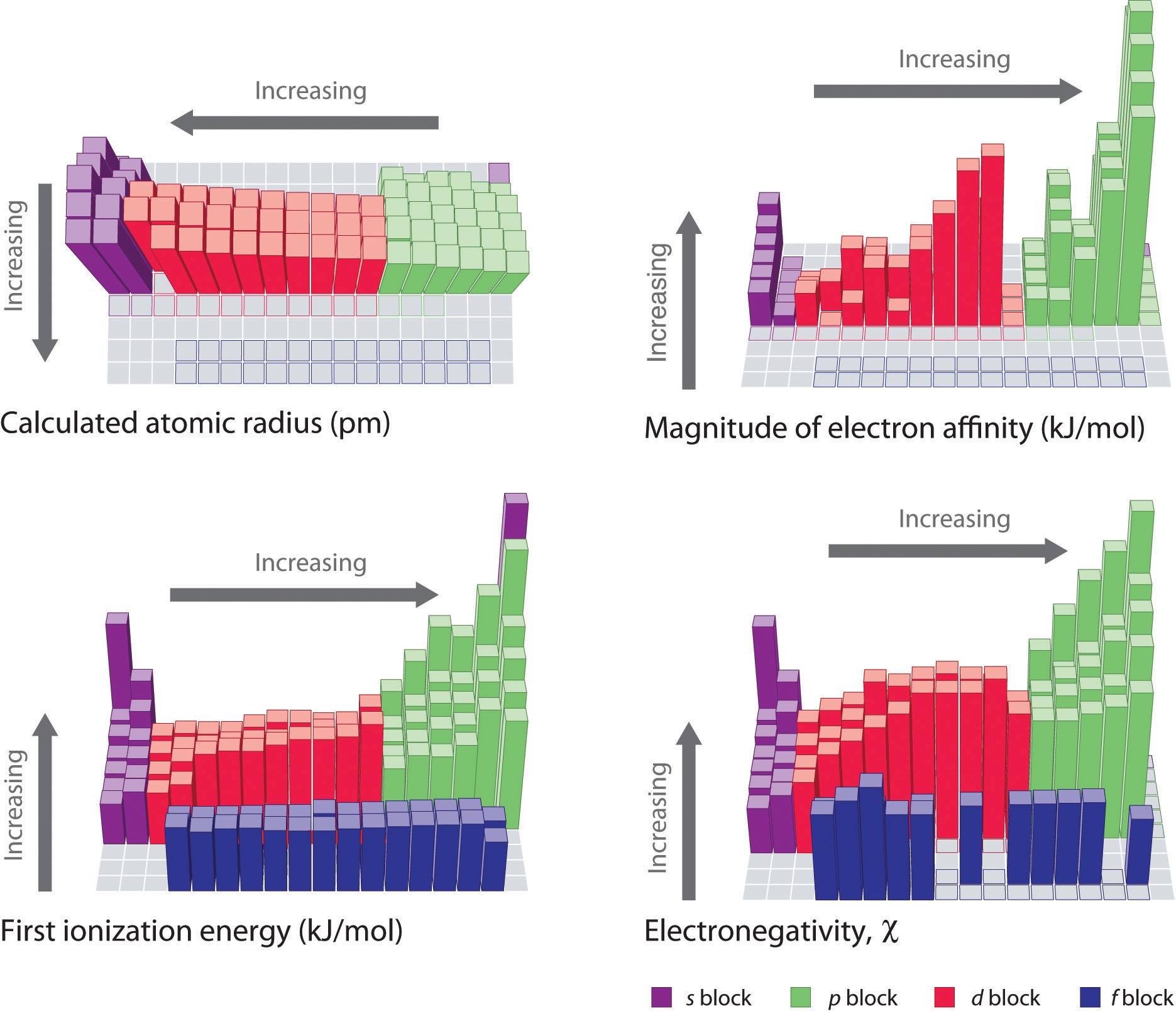
2.9 Periodic Properties of the Elements Chemistry LibreTexts
/PeriodicTableCharge-WBG-56a12db23df78cf772682c37.png)
Periodic Table With Common Ionic Charges
Ionization Ionization Is
6.9 Binary Ionic Compounds and Their Properties Chemistry LibreTexts

Printable Periodic Table With Polyatomic Ions Periodic Table Timeline
7.4 Ionization Energy Chemistry LibreTexts

Calculate The Value Of The Third Ionization Energy Of Lithium How To
Ionization Enthalpy NEET Lab
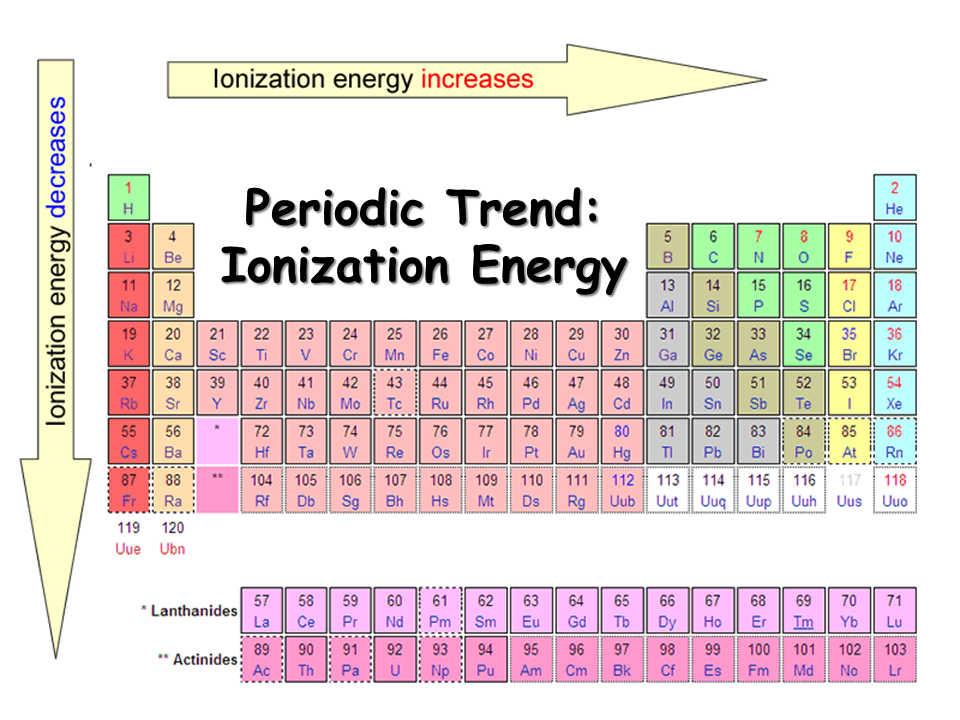
9.9 Periodic Trends Atomic Size, Ionization Energy, and Metallic

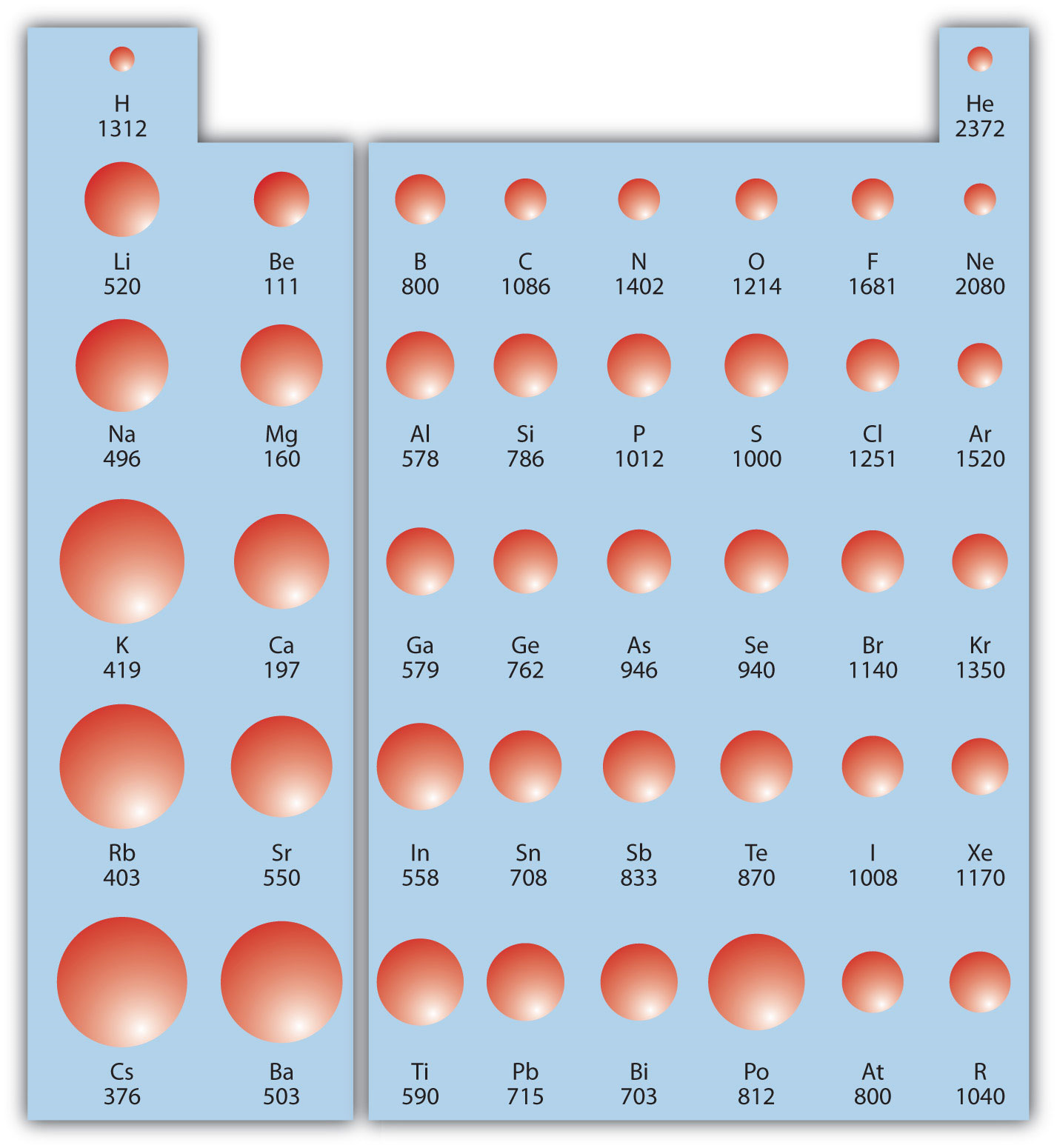
The Parts of the Periodic Table
For Chemistry Students And Teachers:
Also, Learn First & Second Ionization Energies.
First Ionization Energy, Second Ionization Energy As Well As Third Ionization Energy Of The Elements Are Given In This Chart.
The First Molar Ionization Energy Applies To The Neutral Atoms.
Related Post: