Hypertonic Drawing
Hypertonic Drawing - 99k views 4 years ago biology. Web a hypertonic solution has a higher concentration of solutes compared to another solution across a semipermeable membrane. Web cells react differently in hypotonic, isotonic, and hypertonic solutions. Read this study guide to get a deep understanding of these types of solutes. Web hypertonic solution the solution whose osmotic pressure is higher than that of the other is called a hypertonic solution. Web in this video we discuss the three types of osmotic solutions: This type of solution exerts greater osmotic pressure and draws water out of cells placed in it, potentially leading to cell shrinkage or crenation. Students will learn about isotonic, hypertonic, and hypotonic solutions and how these solutions affect the movement of water molecules across the cell membrane. Plant cells in a hypertonic solution can look like a pincushion because of what’s going on inside. Outline the route of administration and appropriate dosing for hypertonic fluids. Web need help in understanding hypotonic vs hypertonic, and isotonic solutions? Hypertonic, hypotonic and isotonic solutions! What is the difference between hypertonic and hypotonic hydration? Web a solution having a higher solute concentration or lower water content than another solution is known as a hypertonic solution (latin ‘hyper’ means ‘over’ or ‘above’). Web a hypertonic solution contains a higher concentration. Web the amount of water outside a cell compared to the inside creates an osmotic pressure gradient which causes water to move. Web hypertonic refers to a solution with higher osmotic pressure than another solution. Web to show students how different concentrations in solutions can affect the cell. Hypotonic solutions, on the other hand, have a lower solute concentration and. Blood cells suspended in a solution containing more than 0.9% (m/v) sodium chloride solution (saline). In an isotonic solution, there is no net flow of water, keeping the cell stable. A hypotonic solution has a lower concentration of solute than another solution, meaning water will flow out of it. What is the difference between hypertonic and hypotonic hydration? Outline the. Web hypertonic, hypotonic, and isotonic solutions. Web a hypertonic solution has a higher concentration of solute than another solution, meaning water will flow into it. Whether a solution is hypertonic or not is measured by comparing the concentration of a solution with another, generally cell sap. Web hypertonic refers to a solution with higher osmotic pressure than another solution. Web. Web when placed in a hypertonic solution, a red blood cell will lose water and undergo crenation (shrivel). Web three terms—hypertonic, hypotonic, and isotonic—are used to describe whether a solution will cause water to move into or out of a cell: Web hypertonic refers to a solution with higher osmotic pressure than another solution. Web to show students how different. Read this study guide to get a deep understanding of these types of solutes. Web need help in understanding hypotonic vs hypertonic, and isotonic solutions? Animal cells tend to do best in an isotonic environment, where the flow of water in and out of the cell is occurring at equal rates. Web hypertonic, hypotonic, and isotonic solutions. Plant cells in. Web when placed in a hypertonic solution, a red blood cell will lose water and undergo crenation (shrivel). In a hypotonic solution, water rushes into the cell causing it to expand or even burst. Web in this video we discuss the three types of osmotic solutions: 99k views 4 years ago biology. Web hypertonic solution the solution whose osmotic pressure. In other words, if there are more solutes outside the cell than inside, water will move out. Web a hypertonic solution causes cells to shrink as water moves out of the cell to balance solute concentrations. Whether a solution is hypertonic or not is measured by comparing the concentration of a solution with another, generally cell sap. Web hypertonic solutions. Web cells react differently in hypotonic, isotonic, and hypertonic solutions. This type of solution exerts greater osmotic pressure and draws water out of cells placed in it, potentially leading to cell shrinkage or crenation. If a cell is placed in a hypertonic solution, there will be a net flow of water out of the cell, and the cell will lose. Outline the route of administration and appropriate dosing for hypertonic fluids. Web hypertonic solutions have a higher solute concentration and cause water to move out of cells, potentially leading to cell shrinkage. Web hypotonic, isotonic and hypertonic solutions (tonicity). Web the amount of water outside a cell compared to the inside creates an osmotic pressure gradient which causes water to. Web when placed in a hypertonic solution, a red blood cell will lose water and undergo crenation (shrivel). This causes water to rush out making the cell wrinkle or shrivel. Hypertonic, hypotonic and isotonic solutions! In this case, water will leave the cell since the cell has a lower osmolarity than the extracellular fluid. Outline the route of administration and appropriate dosing for hypertonic fluids. 99k views 4 years ago biology. Web a hypertonic solution causes cells to shrink as water moves out of the cell to balance solute concentrations. The opposite solution, with a lower concentration or osmolarity, is known as the hypotonic solution. This type of solution exerts greater osmotic pressure and draws water out of cells placed in it, potentially leading to cell shrinkage or crenation. Web cells react differently in hypotonic, isotonic, and hypertonic solutions. Web hypertonic solution the solution whose osmotic pressure is higher than that of the other is called a hypertonic solution. Students will learn about isotonic, hypertonic, and hypotonic solutions and how these solutions affect the movement of water molecules across the cell membrane. Review the adverse effects and contraindications of hypertonic saline and mannitol. Hypertonic, hypotonic and isotonic solutions! A hypotonic solution has a lower concentration of solute than another solution, meaning water will flow out of it. What is the difference between hypertonic and hypotonic hydration?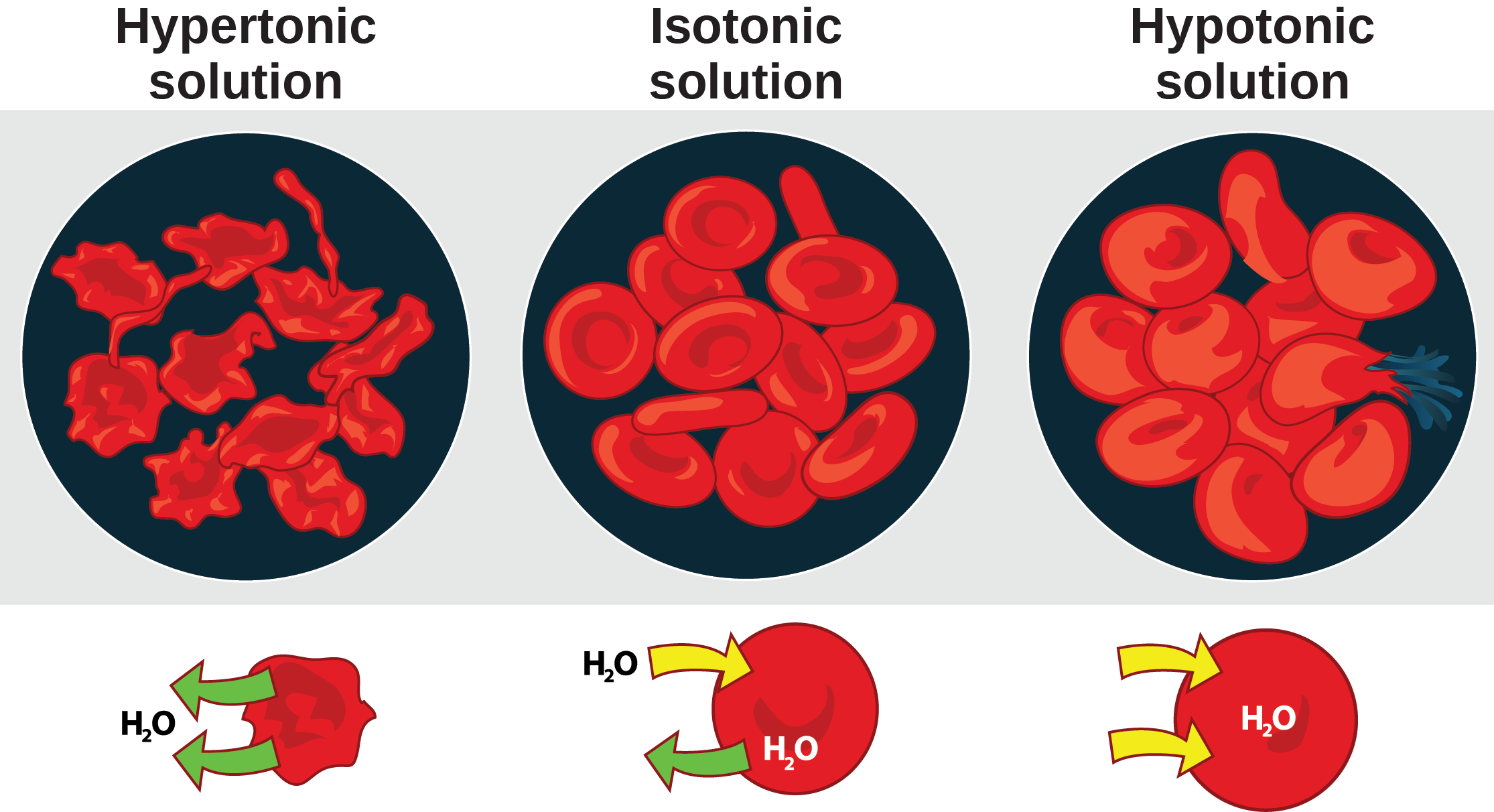
Osmoregulation and Osmotic Balance OpenStax Biology 2e

Types of solutionshypertonic hypotonic and isotonic explained YouTube
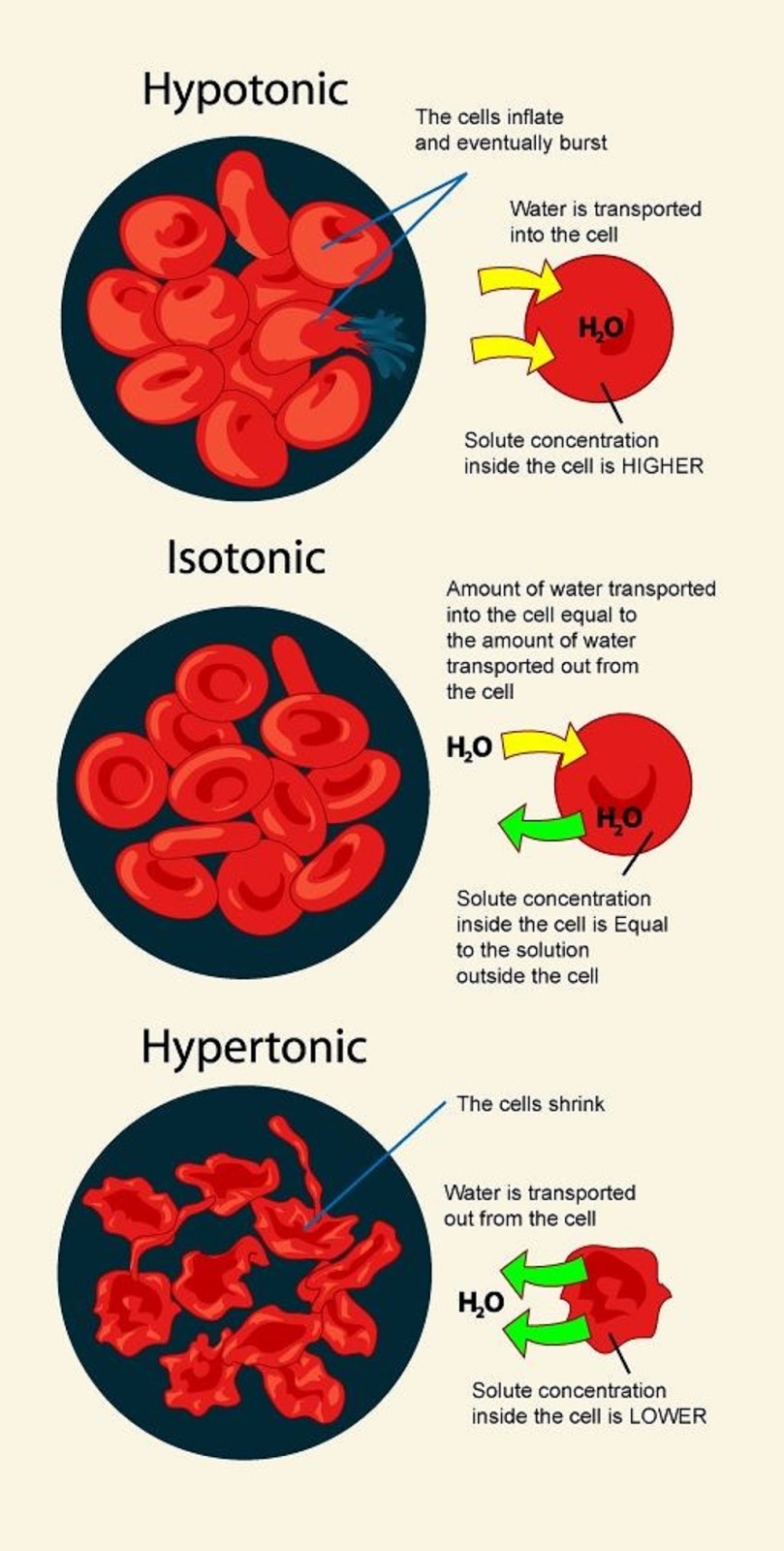
Medical and Health Science Hypotonic, Isotonic, Hypertonic Solution

Hypertonic Hypotonic And Isotonic Cells

Illustration showing the effect of hypotonic, isotonic and hypertonic

effets de hypertonique, hypotonique et istonique solutions à rouge du

Iso, Hypo, and Hypertonic Solutions Diagram Quizlet
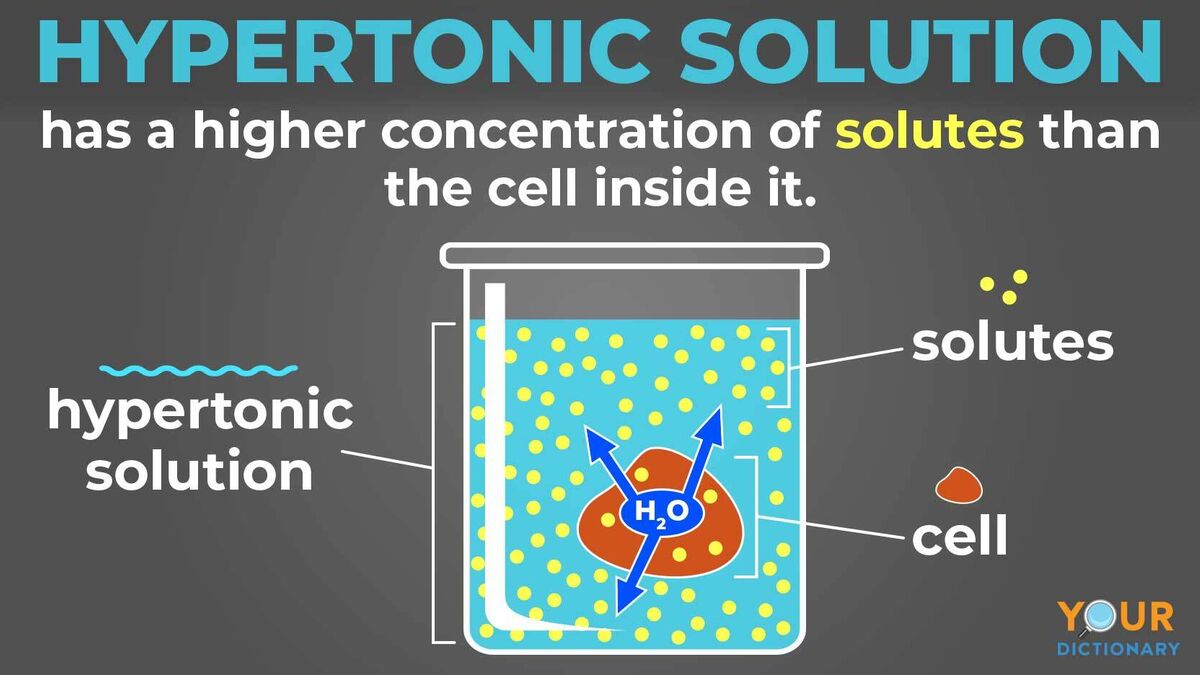
Hypertonic Diagram

Animal Cell In Hypertonic Solution

Hypertonic, Hypotonic, & Isotonic Diagram Quizlet
Web A Hypertonic Solution Contains A Higher Concentration Of Solutes Compared To Another Solution.
In Other Words, If There Are More Solutes Outside The Cell Than Inside, Water Will Move Out.
Whether A Solution Is Hypertonic Or Not Is Measured By Comparing The Concentration Of A Solution With Another, Generally Cell Sap.
In A Hypotonic Solution, Water Rushes Into The Cell Causing It To Expand Or Even Burst.
Related Post: