How Many Orbitals In An Atom Can Have The Designation
How Many Orbitals In An Atom Can Have The Designation - 2.7k views 3 years ago. Web an atomic orbital is a mathematical term in atomic theory and quantum mechanics that describes the position and wavelike behaviour of an electron in an atom. There are four quantum numbers for an orbital: The values are 1, 2, 3,. Web the number of orbitals in an atom with specific designations can be determined using the formula 2l + 1. 1 person found it helpful. Web if i understand your question properly, then. \(d\) orbitals have an orientational. How many orbitals in an atom can have the designation 5p,3dz^2,4d,n=5,n=4?please explain with quantum numbers. For a given set of quantum numbers, each principal shell has a fixed number of subshells, and each subshell has a fixed number of orbitals. Can you please explain more to me? Relative energies of atomic orbitals. Thank you for your help! Possible combinations of quantum numbers. Web if i understand your question properly, then. How many orbitals in an atom can have the designation 5p,3dz^2,4d,n=5,n=4?please explain with quantum numbers. Web each of the 7 4f orbitals accommodates a pair of electrons. A maximum of two electrons, each with its own spin quantum number s, will occupy each of those orbitals. Web how many orbitals in an atom can have each of the following designations:. These electrons are located on the fifth energy level, in the d subshell, i.e. The n quantum number is the principal quantum number, which determines the energy level of the electron. It tells about the position of orbital that is shell number. A maximum of two electrons, each with its own spin quantum number s, will occupy each of those. The quantum mechanical model of the atom tells us that we can only know the location of the electron in terms of its probability. Web n = 5,l = 2. Can you please explain more to me? Electron configurations, the aufbau principle, degenerate orbitals, and. Web each atom has, in general, many orbitals associated with each value of n; How many orbitals in an atom can have the designation 4d ? Web how many orbitals in an atom can have for the following designation? This video solution was recommended by our tutors as helpful for the problem above. The quantum mechanical model of the atom tells us that we can only know the location of the electron in terms. 1 person found it helpful. Web quantum numbers and electron configurations. This video solution was recommended by our tutors as helpful for the problem above. However, this model is not entirely correct. Summary of allowed combinations of quantum numbers. 16 people are viewing now. The values are 1, 2, 3,. As a side note, you can find the number of orbitals that can exist in a subshell by dividing the number of groups in a block by 2. Web each of the 7 4f orbitals accommodates a pair of electrons. Web an atomic orbital is a mathematical term in. However, this model is not entirely correct. How many orbitals in an atom can have each of the following designations: 1s, 2s, 2p 3s, 3p,4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p. Relative energies of atomic orbitals. These orbitals together are sometimes called electron shells. \(d\) orbitals have an orientational. 2.7k views 3 years ago. How many orbitals in an atom can have the designation 4d ? Within each shell of an atom there are some combinations of orbitals. Web how many orbitals in an atom can have the designation? How many orbitals in an atom can have each of the following designations: These electrons are located on the fifth energy level, in the d subshell, i.e. How many orbitals in an atom can have the designation 5$p$ $3 d_{z}, 4 d, n=5, I can do basic problems such as 3p=3, 3d=5, ect.but i really don't know how to figure. Here’s the best way to solve it. Web there are multiple orbitals within an atom. For a given set of quantum numbers, each principal shell has a fixed number of subshells, and each subshell has a fixed number of orbitals. How many atomic orbitals are there for the subshell with [ n = 3, l = 2]? Web the number of orbitals in an atom with specific designations can be determined using the formula 2l + 1. \(d\) orbitals have an orientational. As a side note, you can find the number of orbitals that can exist in a subshell by dividing the number of groups in a block by 2. ( d) n = 3? Can you please explain more to me? Web n = 5,l = 2. Web if i understand your question properly, then. Bismuth is in group 15 of the. Relative energies of atomic orbitals. Web each of the 7 4f orbitals accommodates a pair of electrons. Web how many orbitals in an atom can have the designation. Web how many orbitals in an atom can have the designation?[Solved] 8. How many orbitals in any given atom can have the

Solved How many orbitals in an atom can have each of the

SOLVED How many orbitals in an atom can have designation n = 5? A) 5
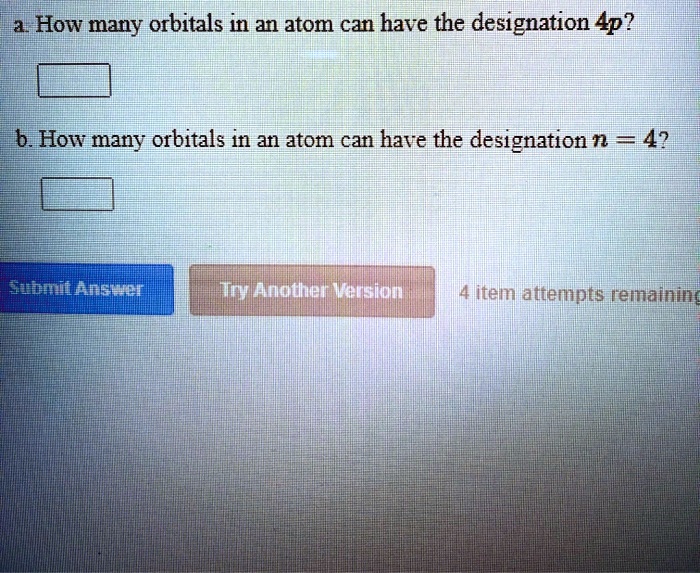
SOLVED a. How many orbitals in an atom can have the designation Ap? b

Atom Electrons, Orbitals, Energy Britannica
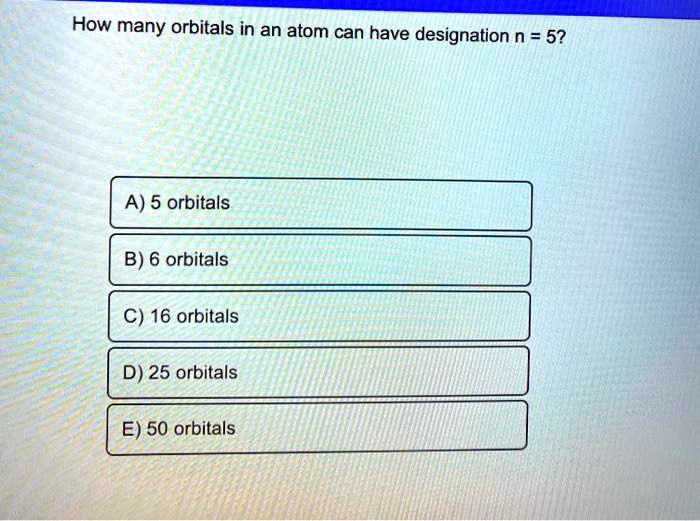
SOLVED How many orbitals in an atom can have designation n = 5? A) 5

PPT Electron Configuration and the Periodic Table PowerPoint
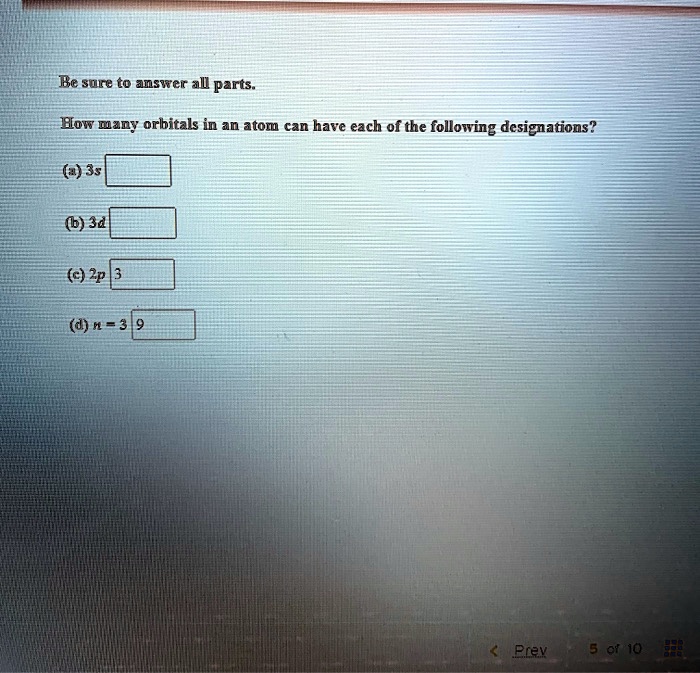
SOLVED How many orbitals in an atom can have each of the following
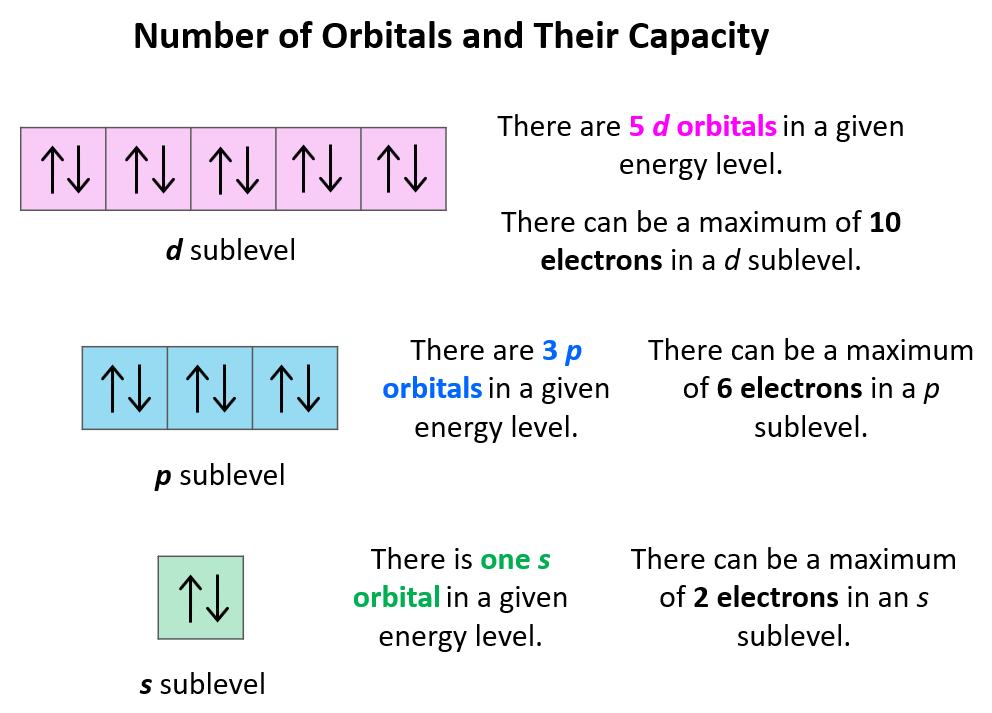
Orbital Diagrams Chemistry Steps

How many orbitals in an atom can have the designation? 5p 3dz2 4d n=5 n
For Example, There Are 3 Orbitals In The 5P Subshell, 5 Orbitals In The 3D Subshell, And 5 Orbitals In The 4D Subshell.
Possible Combinations Of Quantum Numbers.
Bohr's Model Of The Atom Said That Electrons Exists In Specific Orbits With Specific Energies.
These Electrons Are Located On The Fifth Energy Level, In The D Subshell, I.e.
Related Post: