Gspr Checklist Template
Gspr Checklist Template - Web a checklist that manufacturers may complete to demonstrate how they have complied with the gsprs for an ivd, and where the associated evidence can be found, is available. This document is mandatory for the evaluation of the conformity of your medical device per mdr 2017/745 annex i. Download the full article and pdf (including a full gspr flash audit checklist) here. Web therefore, medical device manufacturers should promptly prepare a new checklist for the general safety and performance requirements (gspr) according to. Web for devices with lay persons as end users, spr 22 outlines requirements and considerations for the device’s instructions, variation in user technique, risks of error and. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web eu mdr gspr checklist. Web the general safety and performance requirements check list shall be a controlled document. Web is your company is prepared for your audit and mdr checklist. Web the gspr checklist mention the following points: Designed to align with the standards of regulation (eu) 2017/746 (ivdr) on in vitro. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web the document provides an overview of the general safety and performance requirements (gsprs) that medical device companies must comply with under. Web hi, i saw someone asked for this before, but i am looking for a table/template for the mdr gspr that is free. Web achieve full compliance with your gspr checklist using these top 10 tips. Devices shall be designed and manufactured in such a way as to ensure that the characteristics and performance. Web a checklist that manufacturers may. Web is your company is prepared for your audit and mdr checklist. Web every single section of the eu mdr or ivdr gspr should be assessed in its own right, as it pertains to your medical device. Devices shall be designed and manufactured in such a way as to ensure that the characteristics and performance. Web achieve full compliance with. Web a checklist that manufacturers may complete to demonstrate how they have complied with the gsprs for an ivd, and where the associated evidence can be found, is available. Designed to align with the standards of regulation (eu) 2017/746 (ivdr) on in vitro. Web hi, i saw someone asked for this before, but i am looking for a table/template for. Web the manufacturer shall apply the following principles in the priority order listed: Web a free, comprehensive template to help medical device companies streamline the general safety and performance requirement (gspr) process for mdr compliance. Web the general safety and performance requirements check list shall be a controlled document. Web every single section of the eu mdr or ivdr gspr. Web eu mdr gspr checklist. Web avoid getting overwhelmed and ensure compliance to the eu mdr gsprs using this free checklist and template. Web a checklist that manufacturers may complete to demonstrate how they have complied with the gsprs for an ivd, and where the associated evidence can be found, is available. Web hi, i saw someone asked for this. Web achieve full compliance with your gspr checklist using these top 10 tips. Web for devices with lay persons as end users, spr 22 outlines requirements and considerations for the device’s instructions, variation in user technique, risks of error and. Mention the international standard (preferably harmonized) or the common specification (cs) applicable. Web a free, comprehensive template to help medical. Applicability of the general safety and performance requirements method(s). Web achieve full compliance with your gspr checklist using these top 10 tips. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web the general safety and performance requirements check list shall be a controlled document.. Web for devices with lay persons as end users, spr 22 outlines requirements and considerations for the device’s instructions, variation in user technique, risks of error and. Download the full article and pdf (including a full gspr flash audit checklist) here. Web every single section of the eu mdr or ivdr gspr should be assessed in its own right, as. Web a free, comprehensive template to help medical device companies streamline the general safety and performance requirement (gspr) process for mdr compliance. Web for devices with lay persons as end users, spr 22 outlines requirements and considerations for the device’s instructions, variation in user technique, risks of error and. Web pertieseu mdr annex i, chapter ii, #10.128. Web is your. Web therefore, medical device manufacturers should promptly prepare a new checklist for the general safety and performance requirements (gspr) according to. Web the manufacturer shall apply the following principles in the priority order listed: Applicability of the general safety and performance requirements method(s). Web a free, comprehensive template to help medical device companies streamline the general safety and performance requirement (gspr) process for mdr compliance. Designed to align with the standards of regulation (eu) 2017/746 (ivdr) on in vitro. Web the gspr checklist mention the following points: Web for devices with lay persons as end users, spr 22 outlines requirements and considerations for the device’s instructions, variation in user technique, risks of error and. Web every single section of the eu mdr or ivdr gspr should be assessed in its own right, as it pertains to your medical device. Web eu mdr gspr checklist. Web the general safety and performance requirements check list shall be a controlled document. This document is mandatory for the evaluation of the conformity of your medical device per mdr 2017/745 annex i. Web pertieseu mdr annex i, chapter ii, #10.128. Web a checklist that manufacturers may complete to demonstrate how they have complied with the gsprs for an ivd, and where the associated evidence can be found, is available. Web avoid getting overwhelmed and ensure compliance to the eu mdr gsprs using this free checklist and template. Web digital template for general safety and performance requirements (gspr): The checklist will review all the.
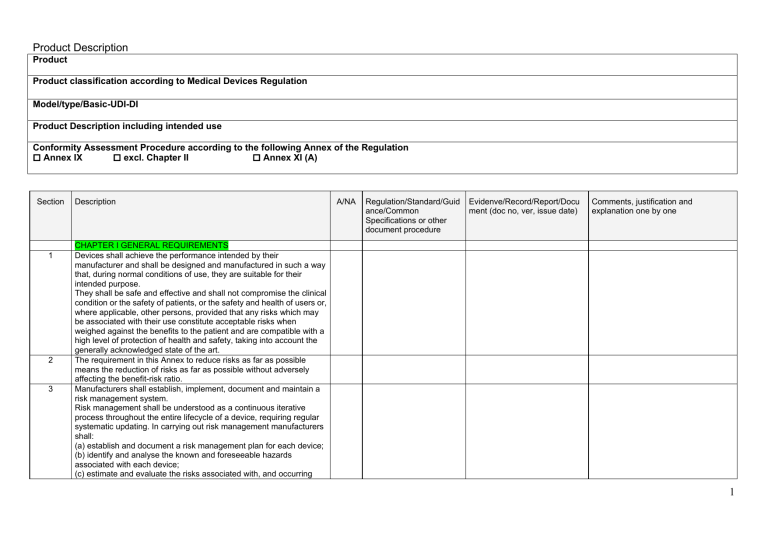
MDR 2017/745 GSPR template Easy Medical Device School
+GSPR+Checklist+-+SCREENSHOT+2+WATERMARK.png?format=2500w)
General Safety And Performance Requirements (GSPR), 54 OFF
+GSPR+Checklist.png?format=1500w)
General Safety and Performance Requirements (GSPR) Checklist — Medical

Comparative table GSPR Essential Requirements (v3) Académie DM Experts
![Free IVDR GSPR Checklist [Template] Trinzo, 45 OFF](https://www.qualio.com/hubfs/Screenshot 2022-09-07 at 12.01.28.png)
Free IVDR GSPR Checklist [Template] Trinzo, 45 OFF
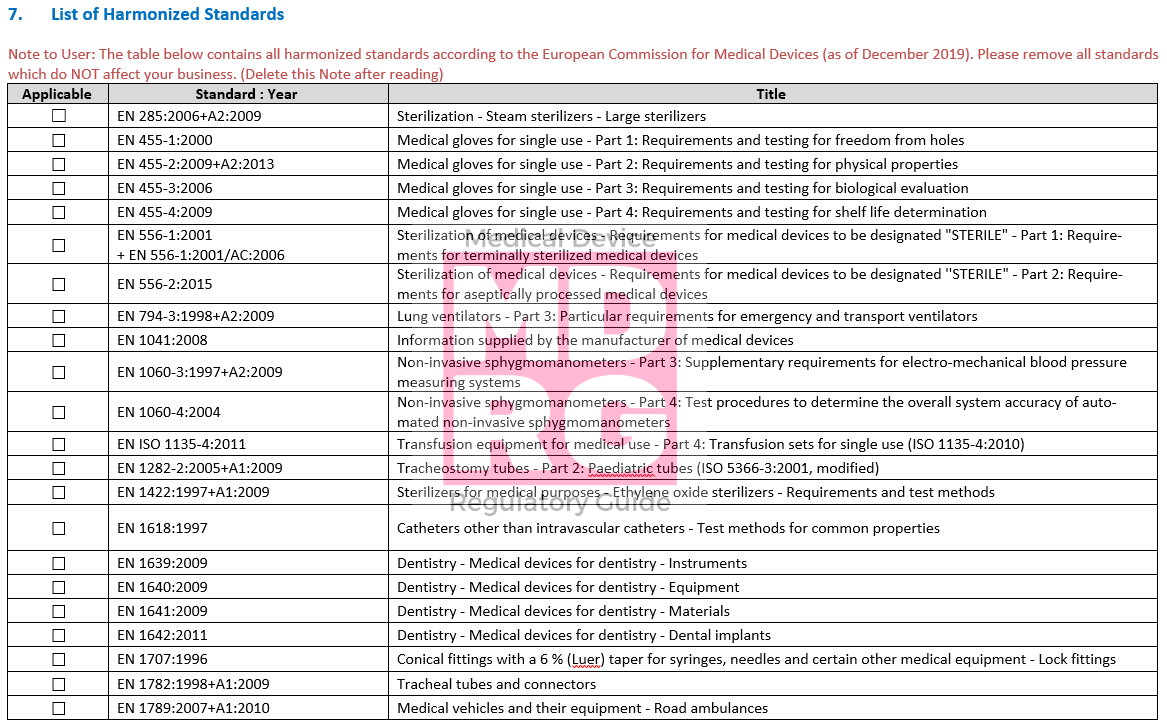
GSPR checklist

Mdr Gspr Checklist Template Printable Word Searches

Australian Essential Principles
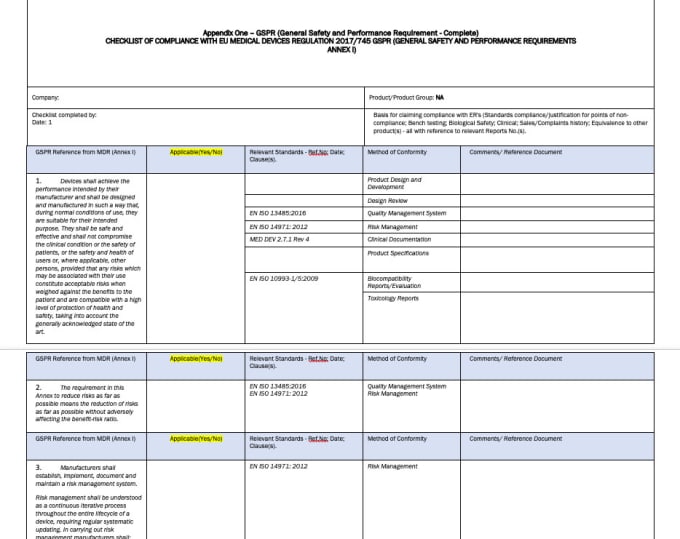
General Safety And Performance Requirements (GSPR), 54 OFF

GSPR Checklist Compliance Top 10 Tips To Achieving, 51 OFF
When A Requirement Applies, A Simple Statement Can.
Mention The International Standard (Preferably Harmonized) Or The Common Specification (Cs) Applicable.
Web Is Your Company Is Prepared For Your Audit And Mdr Checklist.
Web Achieve Full Compliance With Your Gspr Checklist Using These Top 10 Tips.
Related Post: