Group Sequential Design
Group Sequential Design - Web group sequential designs are a type of adaptive clinical trial design that allows for interim analyses and potential early stopping for efficacy or futility based on accumulating data. Web in a (group) sequential study design, samples are analyzed in a sequence where at each stage all the data from earlier stages are combined with the data of the current stage. Web several strategies have been developed to minimize high placebo responses, including the sequential parallel comparison design (spcd). 03, 2017) announced that management will be presenting. Web a general class of bayesian group sequential designs is presented, where multiple criteria based on the posterior distribution can be defined to reflect clinically. Web one group involved a factorial design in which the two conflict types were combined within one trial. Web this paper reviews basic concepts of group sequential analysis and introduces two sas/stat® procedures: See methods, guidance and examples. Web regulatory bodies recommend group sequential designs to protect subjects in a clinical trial and produce results as efficiently as possible. West palm beach, fl 33417. Web regulatory bodies recommend group sequential designs to protect subjects in a clinical trial and produce results as efficiently as possible. Web a general class of bayesian group sequential designs is presented, where multiple criteria based on the posterior distribution can be defined to reflect clinically. As a water quality research and engineering firm. Web derives group sequential clinical trial. Web this paper reviews basic concepts of group sequential analysis and introduces two sas/stat® procedures: Group sequential tests for phase iii clinical trials. See methods, guidance and examples. Web group sequential designs are a type of adaptive clinical trial design that allows for interim analyses and potential early stopping for efficacy or futility based on accumulating data. Web derives group. See methods, guidance and examples. Web for the purposes of this guidance, an adaptive design is defined as a clinical trial design that allows for prospectively planned modifications to one or more aspects of the design. For a study with binary. Web group sequential designs are a type of adaptive clinical trial design that allows for interim analyses and potential. See methods, guidance and examples. Web learn how to use group sequential designs to improve efficiency in pragmatic clinical trials with early outcomes. Work efforts at erd are. Web environmental research and design, inc. 03, 2017) announced that management will be presenting. See methods, guidance and examples. Work efforts at erd are. Group sequential tests for phase iii clinical trials. As a water quality research and engineering firm. In group sequential design, a trial can be stopped prematurely if there are safety or efficacy issues and depending upon the results of the. Web a general class of bayesian group sequential designs is presented, where multiple criteria based on the posterior distribution can be defined to reflect clinically. Web this paper reviews basic concepts of group sequential analysis and introduces two sas/stat® procedures: Web a group sequential design enables repeated analysis of an endpoint for a clinical trial to enable possible early stopping. See methods, guidance and examples. Web the possibility that an individual from the treatment group may not respond to the treatment motivates the use of a mixture distribution for the treatment group. Web group sequential design. Group sequential tests for phase iii clinical trials. For a study with binary. Web group sequential design. Web for the purposes of this guidance, an adaptive design is defined as a clinical trial design that allows for prospectively planned modifications to one or more aspects of the design. Web group sequential designs are a type of adaptive clinical trial design that allows for interim analyses and potential early stopping for efficacy or futility. In group sequential design, a trial can be stopped prematurely if there are safety or efficacy issues and depending upon the results of the. Web in a (group) sequential study design, samples are analyzed in a sequence where at each stage all the data from earlier stages are combined with the data of the current stage. Web group sequential design.. Web in a (group) sequential study design, samples are analyzed in a sequence where at each stage all the data from earlier stages are combined with the data of the current stage. Web derives group sequential clinical trial designs and describes their properties. Web several strategies have been developed to minimize high placebo responses, including the sequential parallel comparison design. Web a general class of bayesian group sequential designs is presented, where multiple criteria based on the posterior distribution can be defined to reflect clinically. Web derives group sequential clinical trial designs and describes their properties. See methods, guidance and examples. Web a group sequential design enables repeated analysis of an endpoint for a clinical trial to enable possible early stopping of a trial for either a positive result, for futility, or for a safety issue. Web regulatory bodies recommend group sequential designs to protect subjects in a clinical trial and produce results as efficiently as possible. Web group sequential designs are a type of adaptive clinical trial design that allows for interim analyses and potential early stopping for efficacy or futility based on accumulating data. Web several strategies have been developed to minimize high placebo responses, including the sequential parallel comparison design (spcd). For a study with binary. Web the possibility that an individual from the treatment group may not respond to the treatment motivates the use of a mixture distribution for the treatment group. Web a general class of bayesian group sequential designs is presented, where multiple criteria based on the posterior distribution can be defined to reflect clinically meaningful decision. Group sequential tests for phase iii clinical trials. 03, 2017) announced that management will be presenting. West palm beach, fl 33417. Web this paper reviews basic concepts of group sequential analysis and introduces two sas/stat® procedures: Web software projects have traditionally been done by the waterfall method, which is a sequential method similar to what is used in construction or any other. Web in a (group) sequential study design, samples are analyzed in a sequence where at each stage all the data from earlier stages are combined with the data of the current stage.
PPT Adaptive Design Methods in Clinical Trials PowerPoint

PPT Optimal Adaptive vs. Optimal Group Sequential Designs PowerPoint

Adaptive vs. Group Sequential Designs in Survival Analysis

PPT Optimal Adaptive vs. Optimal Group Sequential Designs PowerPoint
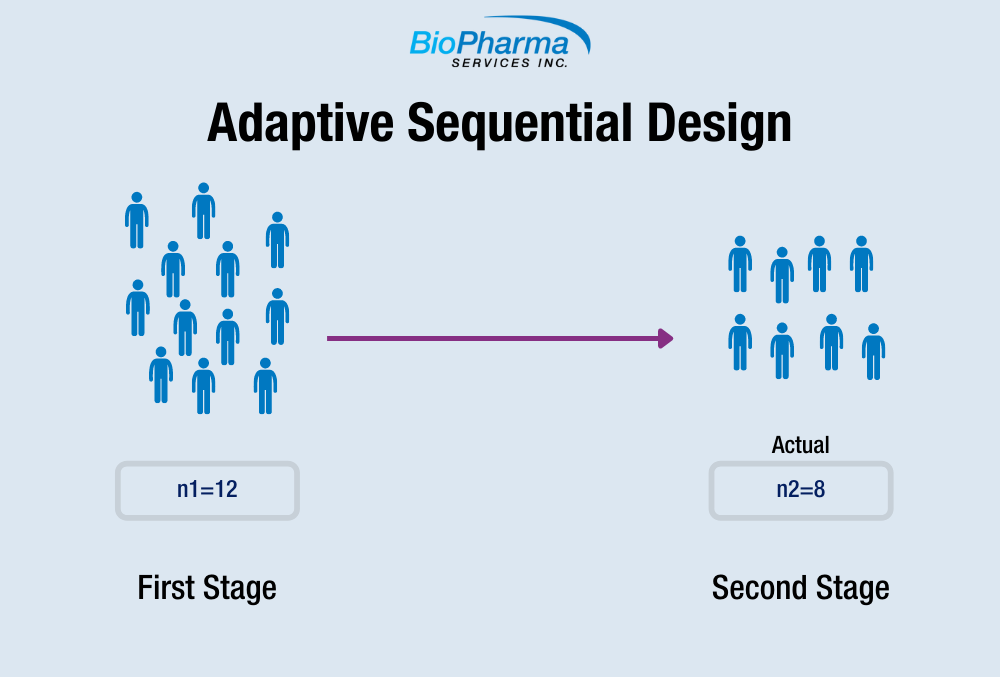
Adaptive Sequential Design in Bioequivalence Trial BioPharma Services

Intro To Adaptive Design

Adaptive designs for clinical trials JLI Blog
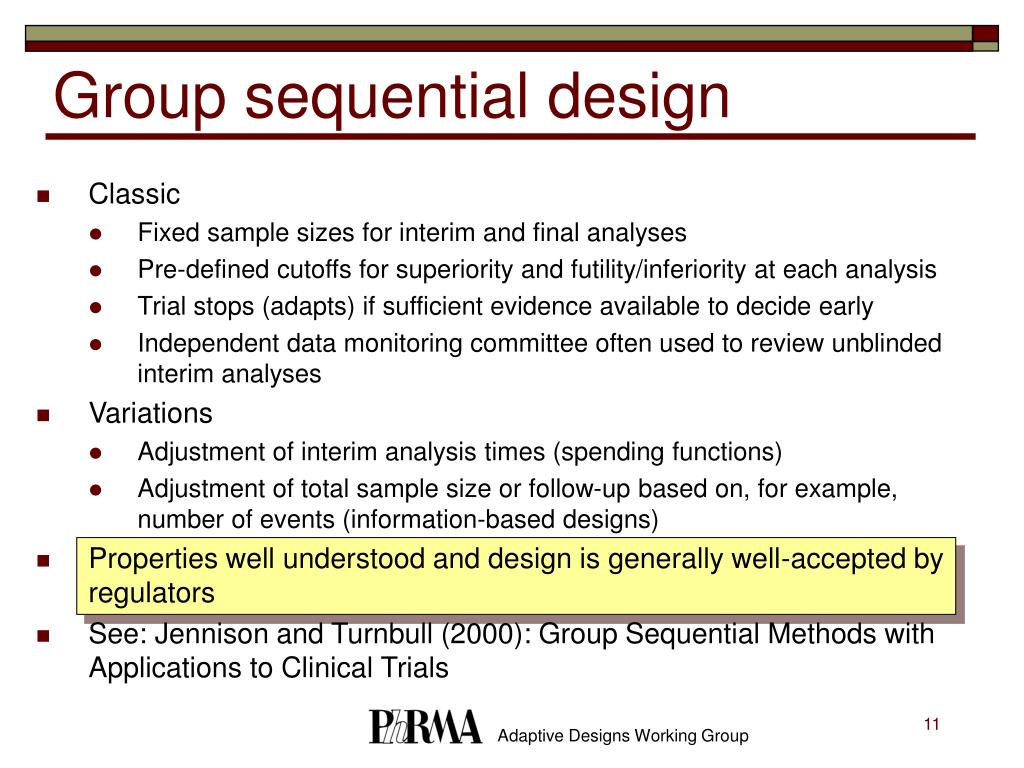
PPT Adaptive Designs Sample size reestimation A review and

Table 3 from Confirmatory adaptive group sequential designs for

PPT Designs for Clinical Trials PowerPoint Presentation, free
This Business Is Not Bbb.
Thus, The Two Tasks Share The Same Relevant Dimension, Which.
Web Learn How To Use Group Sequential Designs To Improve Efficiency In Pragmatic Clinical Trials With Early Outcomes.
As A Water Quality Research And Engineering Firm.
Related Post: