Galvanic Reaction Chart
Galvanic Reaction Chart - The most active metals in the galvanic corrosion chart, like aluminum, zinc, or magnesium, are more likely to corrode when connected to. Web there are four elements necessary for corrosion to occur in a galvanic cell: Web below is a galvanic reaction chart for dissimilar metals. If you're a designer working with exterior metals, you've probably heard of galvanic corrosion. Web view this chart of galvanic compatibility. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. Web galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. Web one of the most important facts that one should know about a metal or an alloy is its reaction with other metals or alloys with which it may be in contact. Use this chart below to better understand what metals will work best together without potential for galvanic corrosion: Web by knowing the relationships of the metals in the series, galvanic compatibility can be determined, preventing the possible harmful effects of galvanic corrosion. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. It includes a chart that shows how different plating materials react. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Web view this chart of galvanic compatibility. The below galvanic corrion chart or anodic index table shows anodic index for different materials. Contact a corrosion specialist to determine the best material for your application. This. The list begins with the more active (anodic) metal and proceeds down. Use this chart below to better understand what metals will work best together without potential for galvanic corrosion: Here the metals are listed in a sequence in which each metal is corroded by all that follow it. Web below is a galvanic reaction chart for dissimilar metals. Web. Web one of the most important facts that one should know about a metal or an alloy is its reaction with other metals or alloys with which it may be in contact. To use the chart, align the metal to be assessed (for the risk of corrosion) in the left column with the contact metal listed in the. Web view. Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. Two metals with different corrosion potentials. There are three conditions that must exist for galvanic corrosion to occur. Water) must connect the two metals on a regular basis. Most architects know enough about it to be. Web view our galvanic series chart, pictures, definitions, and even a mnemonic device! The electrolyte solution creates a “conductive path”. Two metals with different corrosion potentials. Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. We also provide other helpful methods for avoiding galvanic corrosion. Water) must connect the two metals on a regular basis. Web view this chart of galvanic compatibility. Galvanic series / galvanic table. Any metals that are more than 200 millivolts apart in the table will cause the more anodic (higher in the table) The below galvanic corrion chart or anodic index table shows anodic index for different materials. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Web view this chart of galvanic compatibility. Web below is a galvanic reaction chart for dissimilar metals. The list begins with the more active (anodic) metal and proceeds down. The electrolyte solution creates a “conductive. Contact a corrosion specialist to determine the best material for your application. Web below is a galvanic reaction chart for dissimilar metals. Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. Web galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical. Web in general, the farther apart the metals are in the galvanic series, the greater is the corrosion when used together. Web metals listed on the top of the chart (anodic) will corrode faster than the metals on the bottom of the chart (cathodic). Water) must connect the two metals on a regular basis. Web there are four elements necessary. Web in general, the farther apart the metals are in the galvanic series, the greater is the corrosion when used together. Web this article examines how dissimilar metals can lead to galvanic corrosion. The below galvanic corrion chart or anodic index table shows anodic index for different materials. Here the metals are listed in a sequence in which each metal is corroded by all that follow it. Web view our galvanic series chart, pictures, definitions, and even a mnemonic device! So, for example, choosing zinc on zinc would have the lowest risk for corrosion. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Web the galvanic series of metals can be used to determine the likelihood of a galvanic reaction, and galvanic corrosion or bimetallic corrosion, between two different metals in a seawater environment. The following galvanic table lists metals in the order of their relative activity in seawater environment. The most active metals in the galvanic corrosion chart, like aluminum, zinc, or magnesium, are more likely to corrode when connected to. Web view this chart of galvanic compatibility. The electrolyte solution creates a “conductive path”. Web the galvanic corrosion table ranks metals from the most “active” to the least active. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Most architects know enough about it to be dangerous, but what exactly causes this breakdown? It includes a chart that shows how different plating materials react to one another with regard to their galvanic potential.
Galvanic Action Corrosion Prevention Architect's Blog

Galvanic Reaction Chart All Points Fasteners
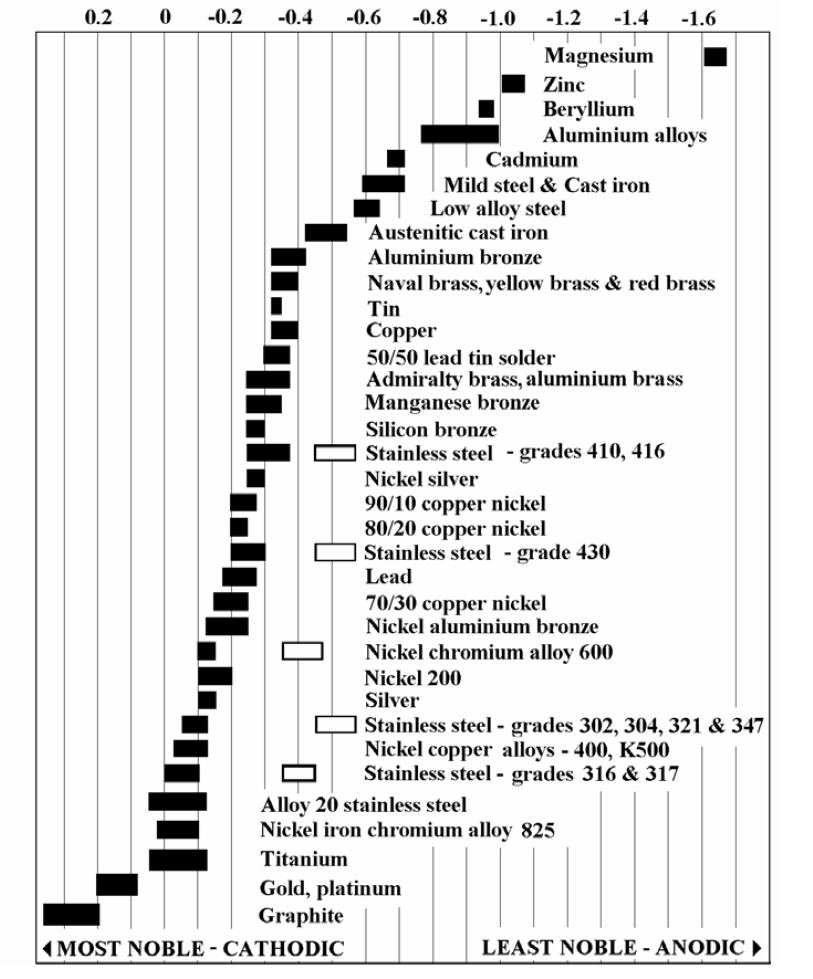
Galvanic Corrosion SSINA
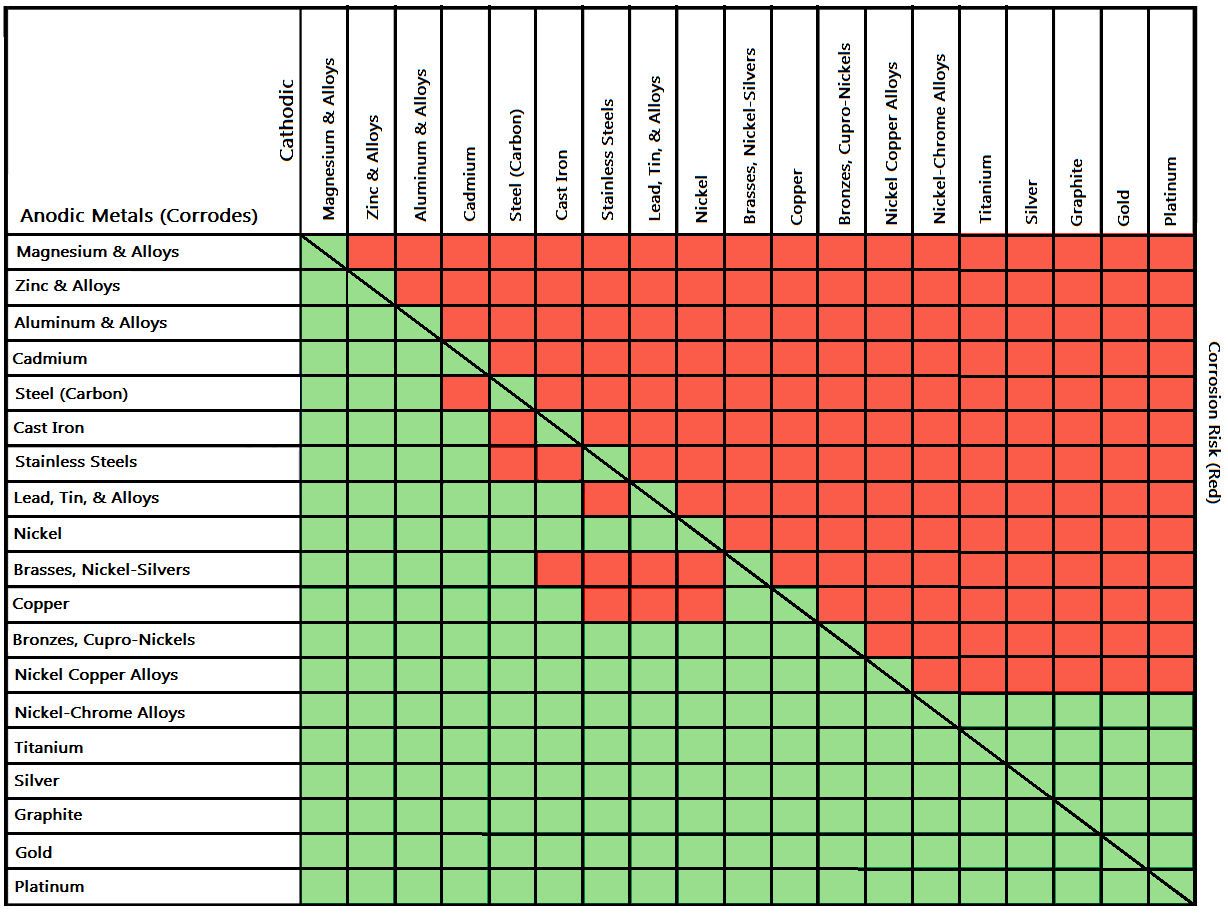
Stainless Steel Galvanic Corrosion Chart
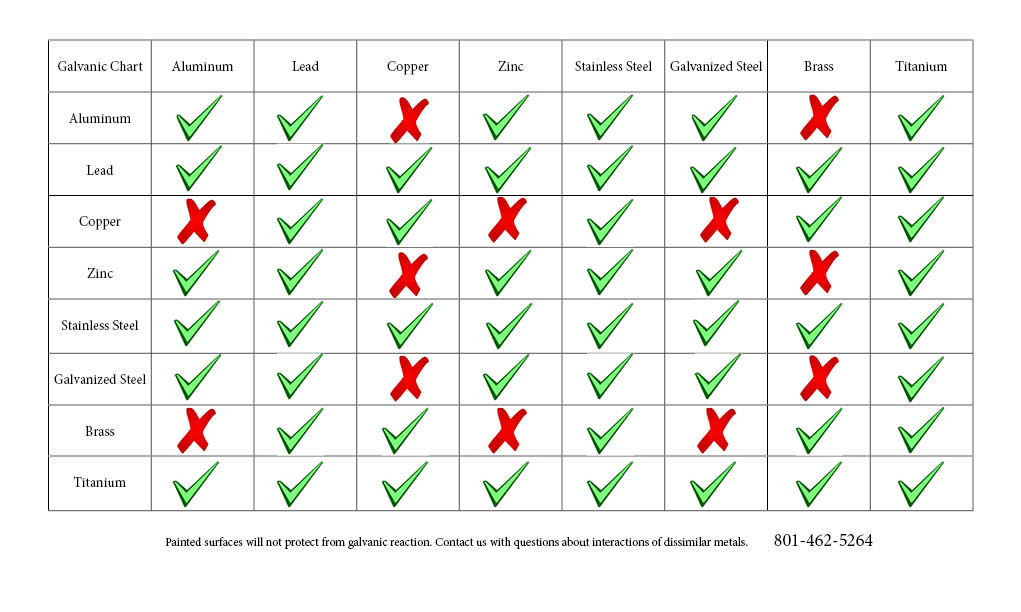
Galvanic Chart For Metals
Galvanic Corrosion Chart PDF Corrosion Electrochemistry
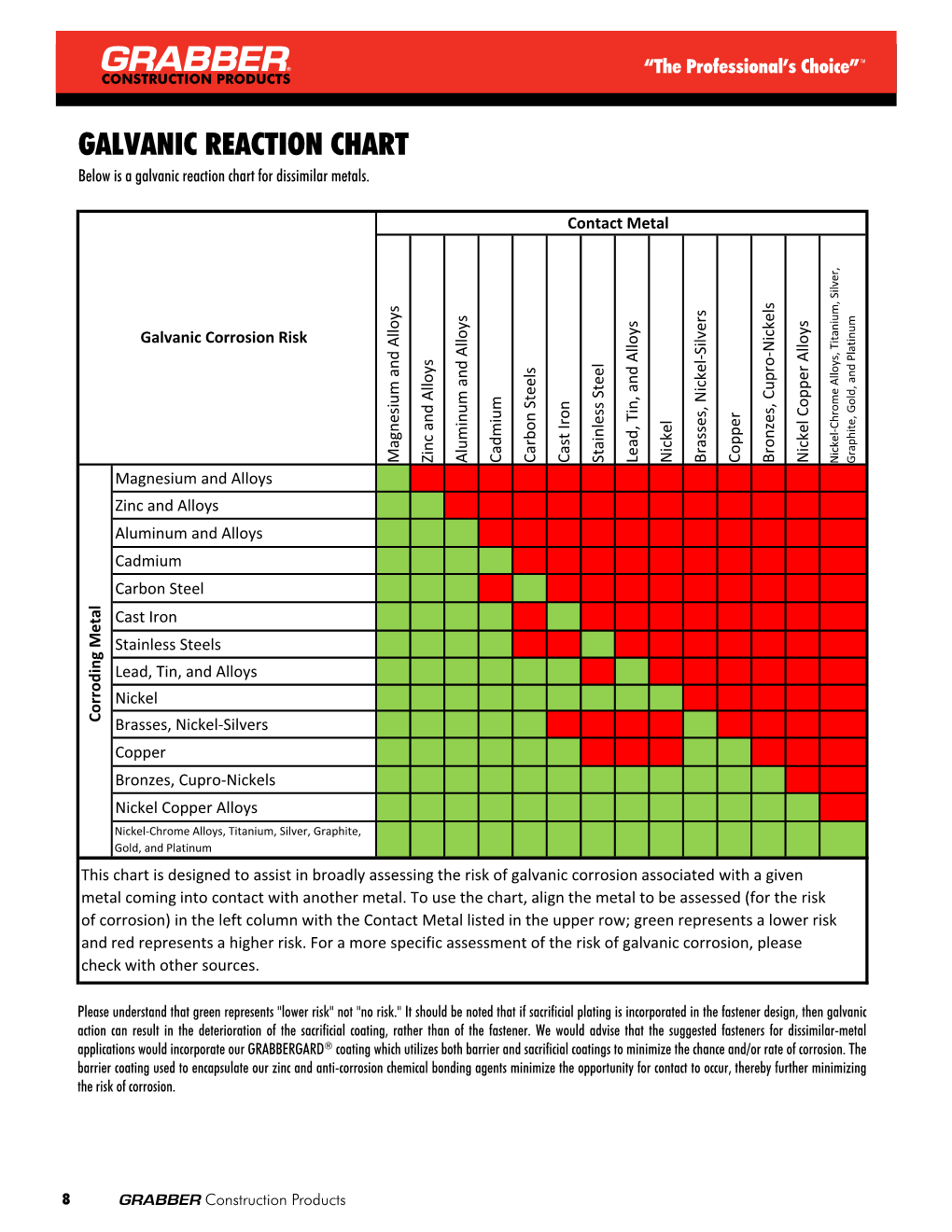
Galvanic Reaction Chart
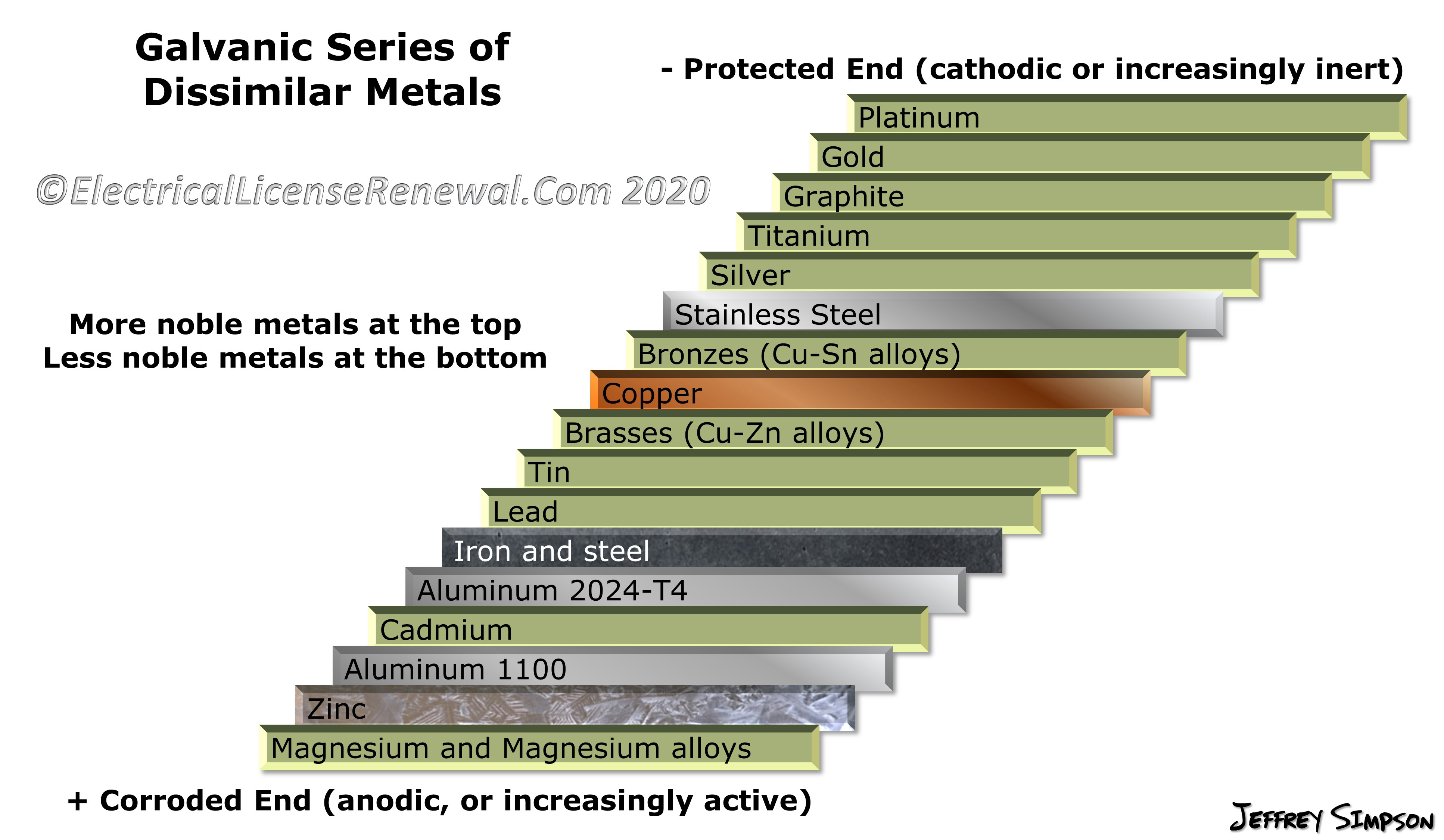
Galvanic Action Chart
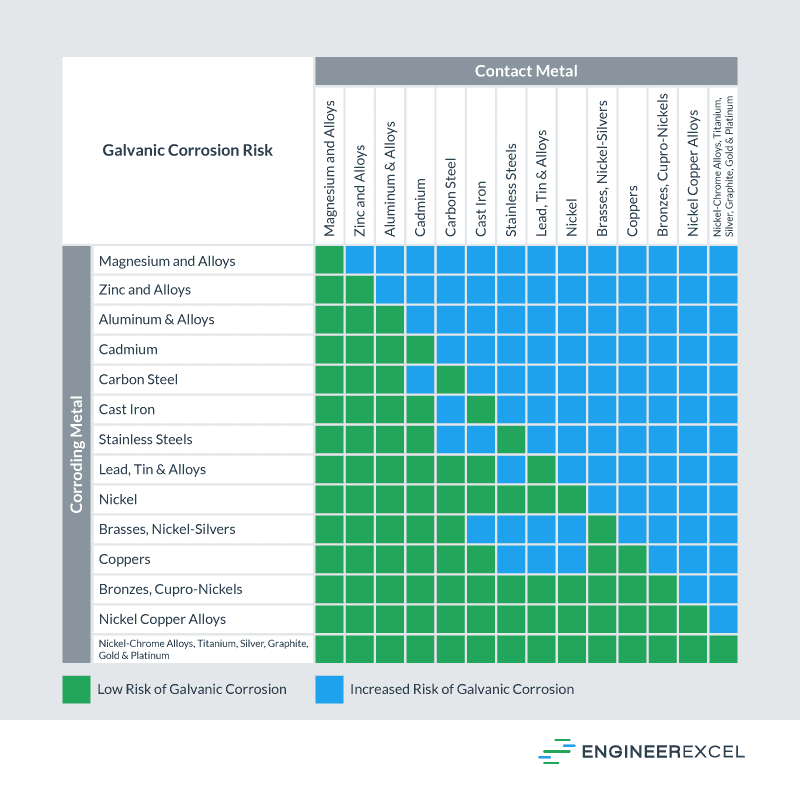
Galvanic Corrosion Between Metals Chart
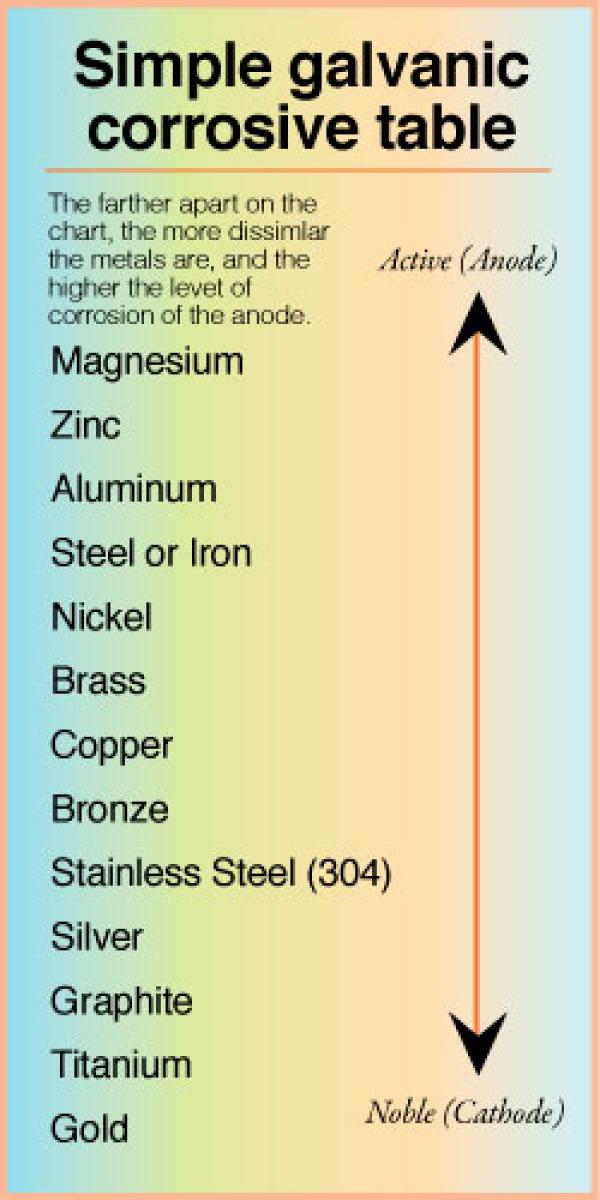
Galvanic Corrosion Chart Metals
Web In Order For Galvanic Corrosion To Occur, Three Elements Are Required.
If You're A Designer Working With Exterior Metals, You've Probably Heard Of Galvanic Corrosion.
The List Begins With The More Active (Anodic) Metal And Proceeds Down.
Web By Knowing The Relationships Of The Metals In The Series, Galvanic Compatibility Can Be Determined, Preventing The Possible Harmful Effects Of Galvanic Corrosion.
Related Post:
