Free Energy Of Formation Chart
Free Energy Of Formation Chart - The partial pressure of any gas involved in the reaction is 0.1 mpa. Web standard heats and free energies of formation and absolute entropies of elements and inorganic compounds The change in free energy represents a. Web at constant temperature and pressure, the change in gibbs free energy is defined as δ g = δ h − t δ s. The figures include nomographs for equilibrium partial pressures. Web the nist chemistry webbook contains: 3 top dividend stocks to buy now. The executive board of the international monetary fund (imf) concluded the article iv consultation [1] with the people’s republic of china. Web in thermodynamics, the gibbs free energy (or gibbs energy as the recommended name; Symbol ) is a thermodynamic potential that can be used to calculate the maximum. The executive board of the international monetary fund (imf) concluded the article iv consultation [1] with the people’s republic of china. Web in thermodynamics, the gibbs free energy (or gibbs energy as the recommended name; The figures include nomographs for equilibrium partial pressures. Calculate the standard free energy change for a process using standard free energies of formation. Web the. Web standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+ aq 105.79 72.7 agcl s. Web at constant temperature and pressure, the change in gibbs free energy is defined as δ g = δ h − t δ s. These are the free energy changes for a. Calculate free energy change for a process using free energies of formation for its reactants and. Reed, free energy of formation of binary compounds,. The concentrations of all aqueous solutions are 1 m. Year to date, platinum is down 1.39%, as of 9 a.m. These values are valid for the temperature 25 c. Web the nist chemistry webbook contains: All standard state, 25 °c and 1 bar (written to 1 decimal place). Calculate free energy change for a process using free energies of formation for its reactants and. The partial pressure of any gas involved in the reaction is 0.1 mpa. The concentrations of all aqueous solutions are 1 m. Mallard, eds, nist chemistry webbook, nist. 3 top dividend stocks to buy now. Is negative, a process will proceed spontaneously and is. Symbol ) is a thermodynamic potential that can be used to calculate the maximum. Reed, free energy of formation of binary compounds,. Thermochemical data for over 7000 organic and small inorganic compounds: Web the nist chemistry webbook contains: Web calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; Secure decades of passive income: The partial pressure of any gas involved in the reaction is 0.1 mpa. Is negative, a process will proceed spontaneously and is. Web define gibbs free energy, and describe its relation to spontaneity; Web standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+ aq 105.79 72.7 agcl s. The change in free energy represents a. Web the change in free. Web definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and gibbs free energy of formation, as well. Explain how temperature affects the spontaneity of some. The change in free energy represents a. Year to date, platinum is down 1.39%, as of 9 a.m. All standard state, 25 °c. 3 top dividend stocks to buy now. Web standard free energies of formation. Web the nist chemistry webbook contains: Web in thermodynamics, the gibbs free energy (or gibbs energy as the recommended name; All standard state, 25 °c and 1 bar (written to 1 decimal place). Calculate free energy change for a process using free energies of formation for its reactants and. Web similar to enthalpy, thermodynamics tables contain an entry for the standard free energy of formation ( d g f º). The figures include nomographs for equilibrium partial pressures. Web the standard free energy of formation of a substance is the free energy change. The figures include nomographs for equilibrium partial pressures. Web standard free energies of formation. Web similar to enthalpy, thermodynamics tables contain an entry for the standard free energy of formation ( d g f º). Reed, free energy of formation of binary compounds,. The change in free energy represents a. Calculate the standard free energy change for a process using standard free energies of formation. Web calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; Web the standard free energy of formation of a substance is the free energy change that occurs when 1 mole of the substance is formed from its constituent elements in their. Is negative, a process will proceed spontaneously and is. Web define gibbs free energy, and describe its relation to spontaneity; Thermochemical data for over 7000 organic and small inorganic compounds: Web free energy of formation is negative for most metal oxides, and so the diagram is drawn with ∆g=0 at the top of the diagram, and the values of ∆g shown are all negative. Explain how temperature affects the spontaneity of some. All standard state, 25 °c and 1 bar (written to 1 decimal place). Web at constant temperature and pressure, the change in gibbs free energy is defined as δ g = δ h − t δ s. The concentrations of all aqueous solutions are 1 m.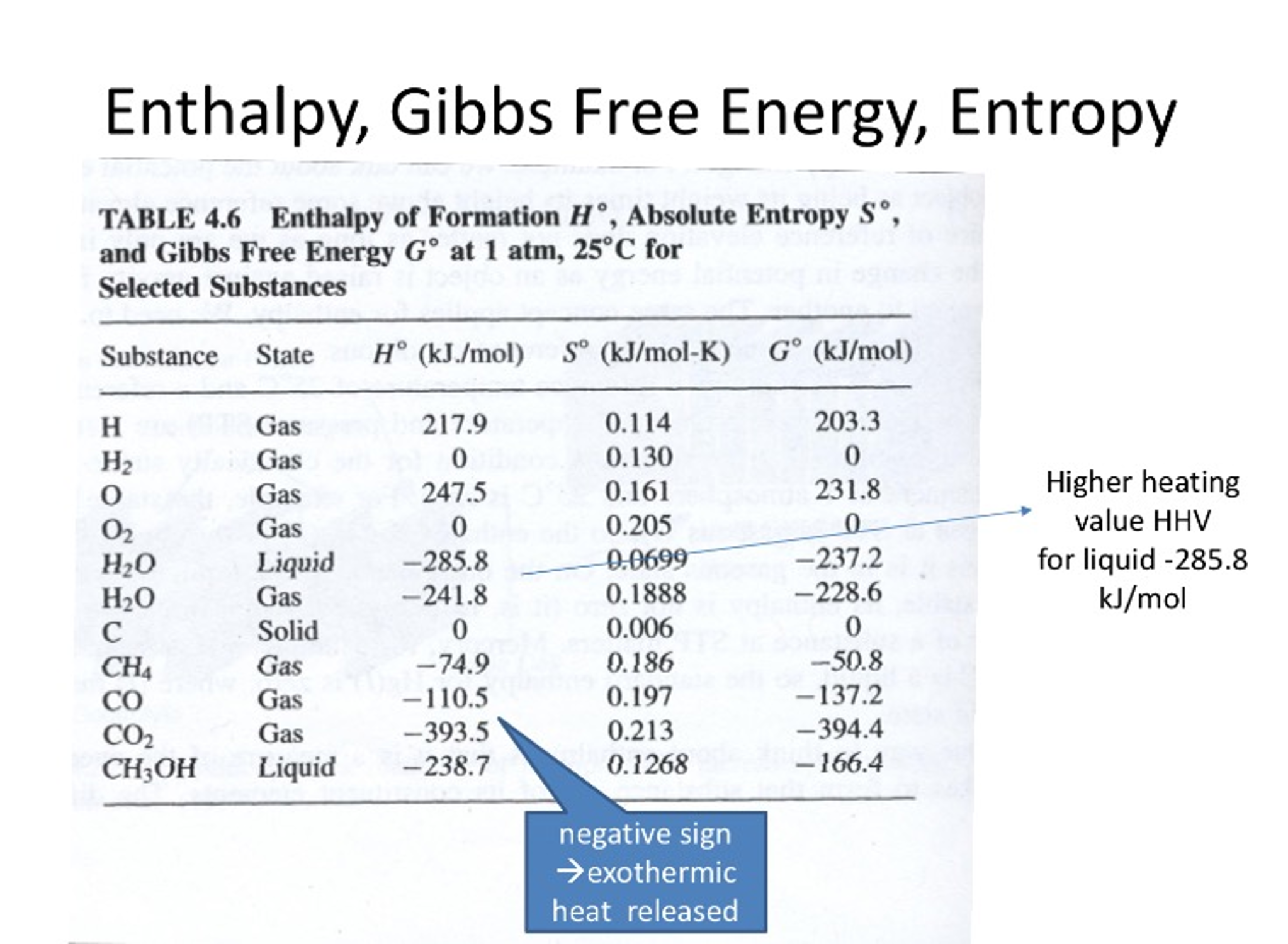
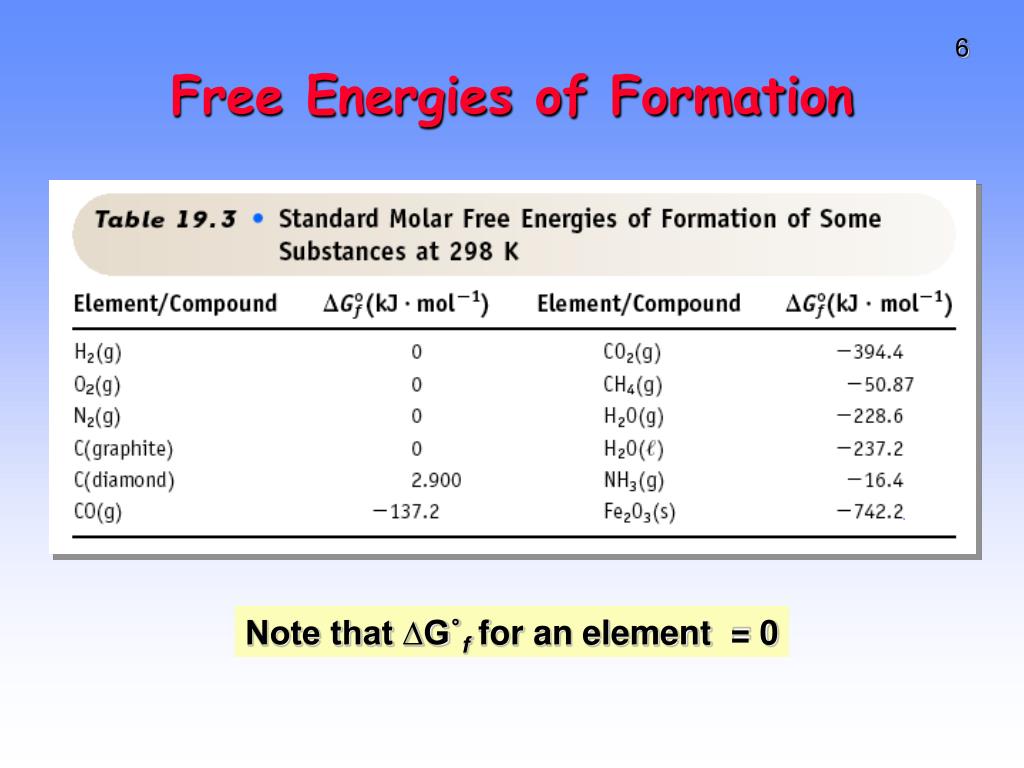
Free Energy Of Formation Table
Standard Gibbs Free Energy Of Formation Table slideshare
PPT Gibbs Free Energy, G PowerPoint Presentation, free download ID

Standard Gibbs free energy of formation, standard enthalpy of formation
Free Energy Of Formation Chart A Visual Reference of Charts Chart Master

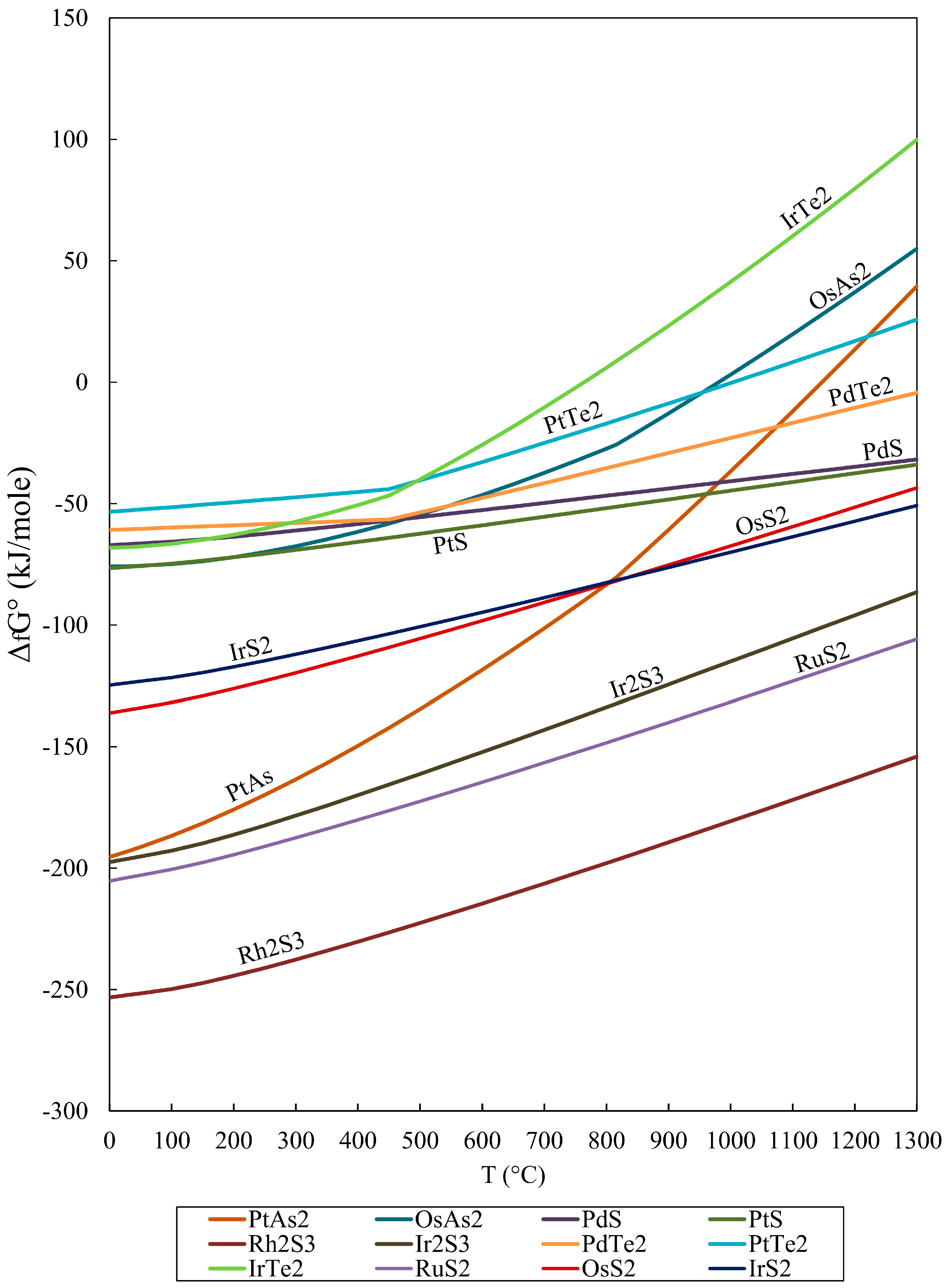
Gibbs free energy of formation values for various oxides. Download

Standard Gibbs Free Energy Of Formation Table slideshare

Standard Gibbs Free Energy Of Formation Table slideshare

Standard Gibbs Free Energy Table vrogue.co

Standard Gibbs Free Energy Of Formation Table slideshare
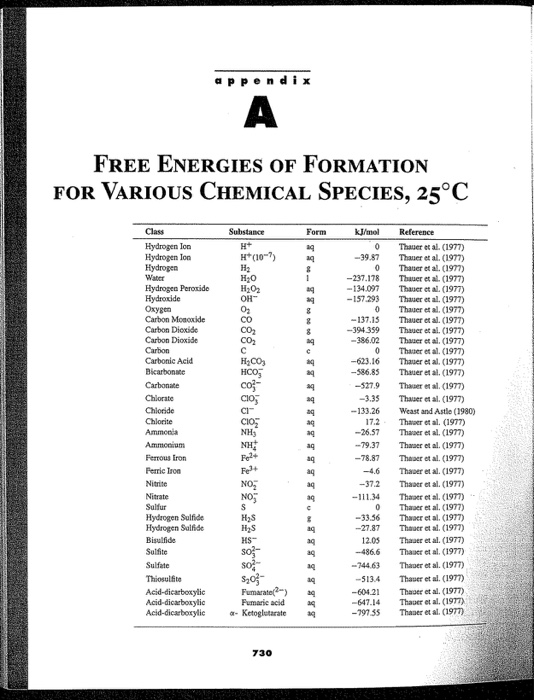
Web Standand Enthalpies Of Formation & Standard Entropies Of Common Compounds Substance State ∆H F S (Kjmol) (Jmol·k) Ag S 0 42.6 Ag+ Aq 105.79 72.7 Agcl S.
Symbol ) Is A Thermodynamic Potential That Can Be Used To Calculate The Maximum.
3 Top Dividend Stocks To Buy Now.
Mallard, Eds, Nist Chemistry Webbook, Nist.
Related Post: