Enthalpy Entropy Chart
Enthalpy Entropy Chart - Web the absolute entropy of a substance at any temperature above 0 k must be determined by calculating the increments of heat q required to bring the substance from 0 k to the temperature of interest, and then summing the ratios q/t. Why was this calculator made? In general, it is a relationship between enthalpy (measure of the energy of a thermodynamic system), air temperature, and moisture content. Web the figures and tables below shows how water enthalpy and entropy changes with temperature (°c and °f) at water saturation pressure (which for practicle use, gives the same result as atmospheric pressure at temperatures < 100 °c (212°f)). These lines extend at an angle from the saturated vapor line. Web the diagram below can be used to determine enthalpy versus entropy of water and steam. Web where enthalpy is a measurement of energy potential, entropy measures the randomness of energy with relation to heat. Denoted as δs, the change of entropy suggests that time itself is asymmetric with respect to order of an isolated system, meaning: When energy is added) or decreases (because energy is given off) is a crucial factor that determines whether a. Some important terms related to enthalpy: Denoted as δs, the change of entropy suggests that time itself is asymmetric with respect to order of an isolated system, meaning: Web the mollier diagram is a chart on which enthalpy (h) versus entropy (s) is plotted. In general, it is a relationship between enthalpy (measure of the energy of a thermodynamic system), air temperature, and moisture content. Enthalpy. Some important terms related to enthalpy: These lines extend at an angle from the saturated vapor line. Why was this calculator made? E= u + pv where, e is the enthalpy. In general, it is a relationship between enthalpy (measure of the energy of a thermodynamic system), air temperature, and moisture content. Web the figures and tables below shows how water enthalpy and entropy changes with temperature (°c and °f) at water saturation pressure (which for practicle use, gives the same result as atmospheric pressure at temperatures < 100 °c (212°f)). When energy is added) or decreases (because energy is given off) is a crucial factor that determines whether a. Web enthalpy. Understanding enthalpy and entropy clarify what these differences mean and when to use each measurement. This definition of enthalpy can be expressed, mathematically, as follows: Web the figures and tables below shows how water enthalpy and entropy changes with temperature (°c and °f) at water saturation pressure (which for practicle use, gives the same result as atmospheric pressure at temperatures. Web the mollier diagram is a graph used in thermodynamics to visualize the relationships between temperature, pressure, specific volume, enthalpy, and entropy of a substance. H = u + p.v eq. The relationship between enthalpy and entropy: Web where enthalpy is a measurement of energy potential, entropy measures the randomness of energy with relation to heat. Denoted as δs, the. Enthalpy is defined as the total heat content or total useful energy of a substance. Web enthalpy ( / ˈɛnθəlpi / ⓘ) is the sum of a thermodynamic system 's internal energy and the product of its pressure and volume. Two kinds of experimental measurements are needed: Web definition and explanation of the terms standard state and standard enthalpy of. In general, it is a relationship between enthalpy (measure of the energy of a thermodynamic system), air temperature, and moisture content. The symbol for enthalpy is “h.” enthalpy is also considered to be the sum of internal energy “u” and flow energy (or flow work) p.v. E= u + pv where, e is the enthalpy. Web the diagram below can. Web the diagram below can be used to determine enthalpy versus entropy of water and steam. These lines extend at an angle from the saturated vapor line. H = u + p.v eq. Some important terms related to enthalpy: Two kinds of experimental measurements are needed: When energy is added) or decreases (because energy is given off) is a crucial factor that determines whether a. In general, it is a relationship between enthalpy (measure of the energy of a thermodynamic system), air temperature, and moisture content. Web the diagram below can be used to determine enthalpy versus entropy of water and steam. Web the mollier diagram. Two kinds of experimental measurements are needed: Web the mollier diagram is a graph used in thermodynamics to visualize the relationships between temperature, pressure, specific volume, enthalpy, and entropy of a substance. Understanding enthalpy and entropy clarify what these differences mean and when to use each measurement. Denoted as δs, the change of entropy suggests that time itself is asymmetric. H = u + p.v eq. Two kinds of experimental measurements are needed: U is the internal energy of a system. Web the diagram below can be used to determine enthalpy versus entropy of water and steam. When energy is added) or decreases (because energy is given off) is a crucial factor that determines whether a. Web the mollier diagram is a graph used in thermodynamics to visualize the relationships between temperature, pressure, specific volume, enthalpy, and entropy of a substance. Some important terms related to enthalpy: Web where enthalpy is a measurement of energy potential, entropy measures the randomness of energy with relation to heat. Why was this calculator made? Web the heat that passes into or out of the system during a reaction is the enthalpy change. Web the figures and tables below shows how water enthalpy and entropy changes with temperature (°c and °f) at water saturation pressure (which for practicle use, gives the same result as atmospheric pressure at temperatures < 100 °c (212°f)). Understanding enthalpy and entropy clarify what these differences mean and when to use each measurement. Whether the enthalpy of the system increases (i.e. This definition of enthalpy can be expressed, mathematically, as follows: E= u + pv where, e is the enthalpy. Denoted as δs, the change of entropy suggests that time itself is asymmetric with respect to order of an isolated system, meaning: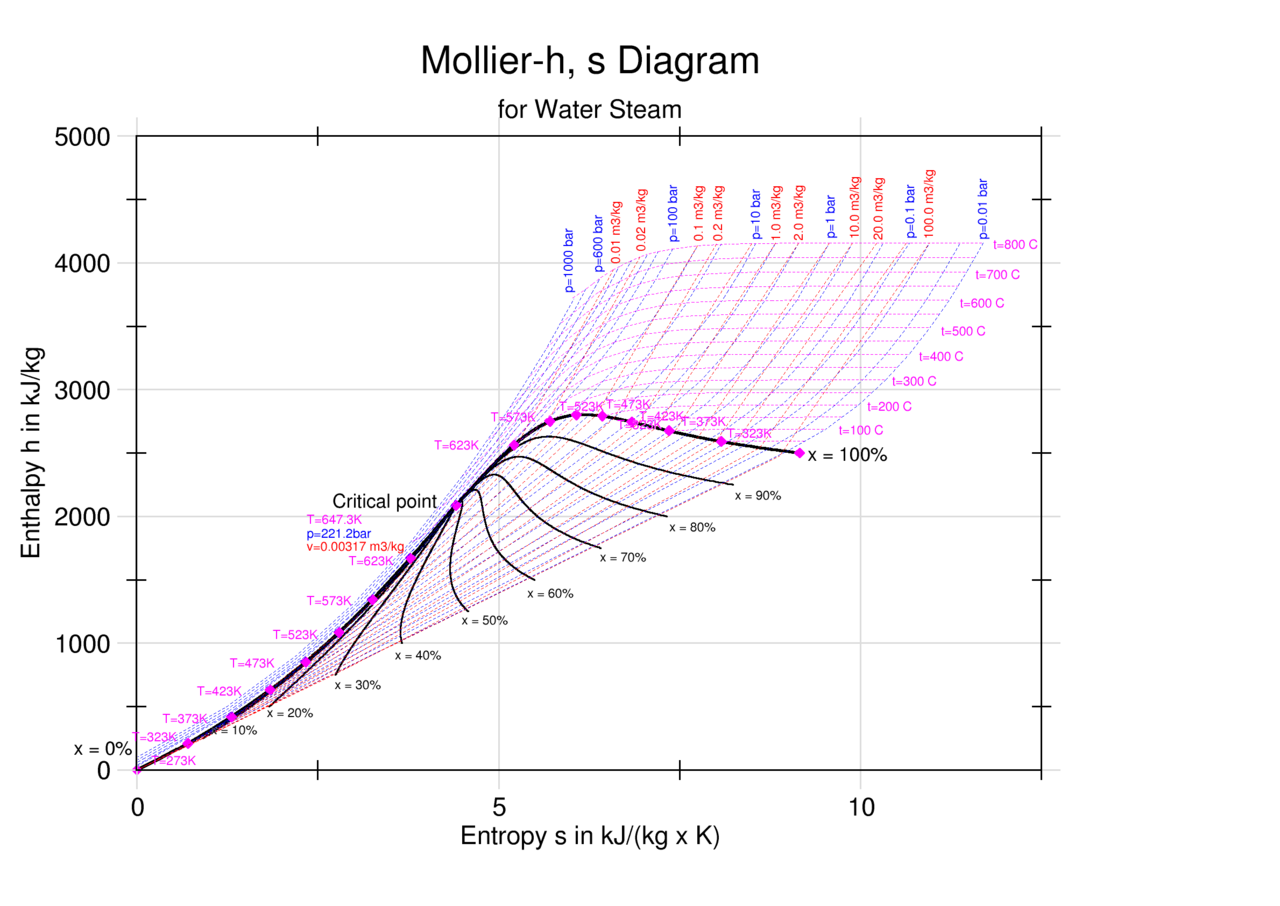
Enthalpy Entropy (hs) or Mollier Diagram
Enthalpy And Entropy Pdf flavilen
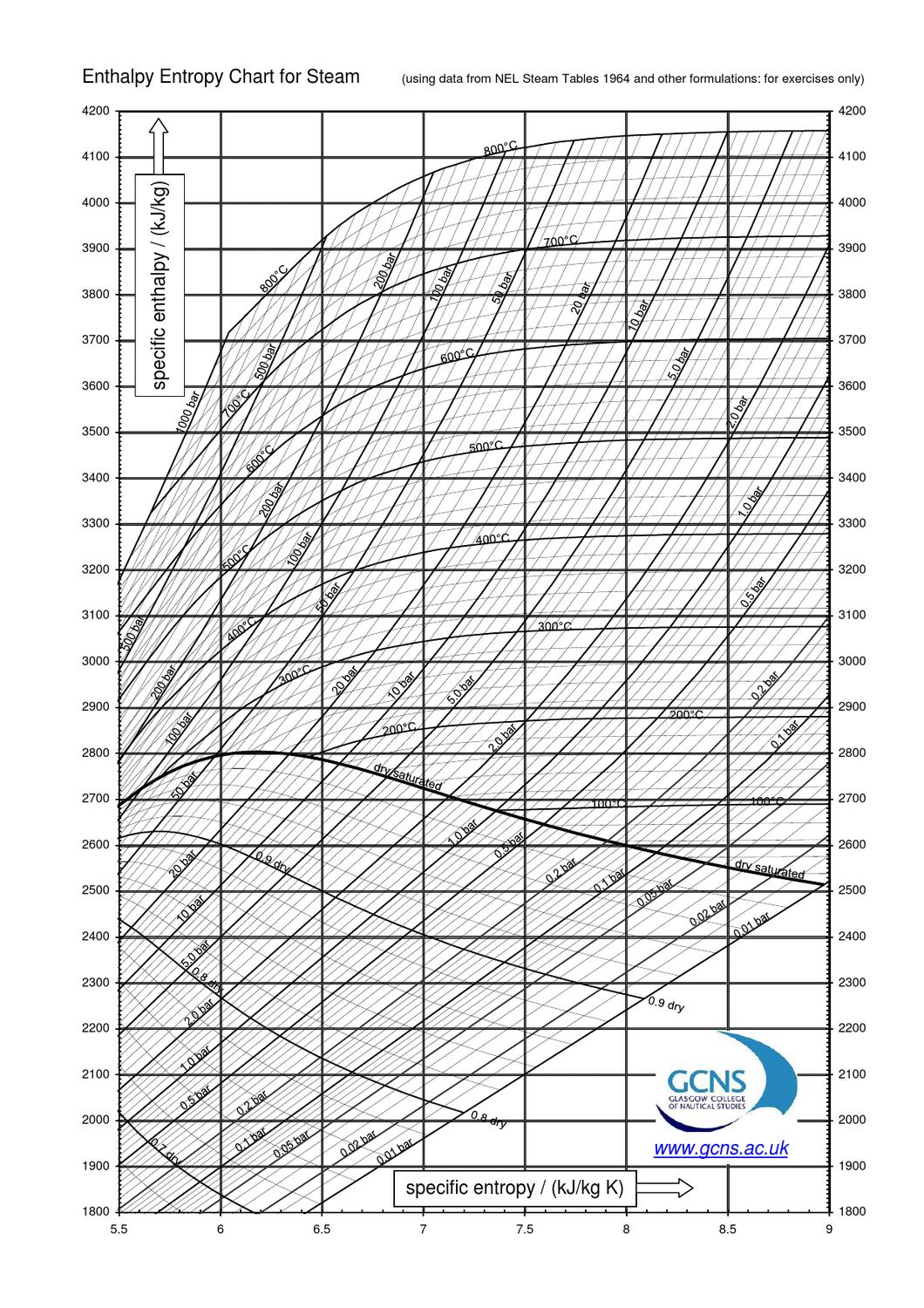
Enthalpy And Entropy Chart

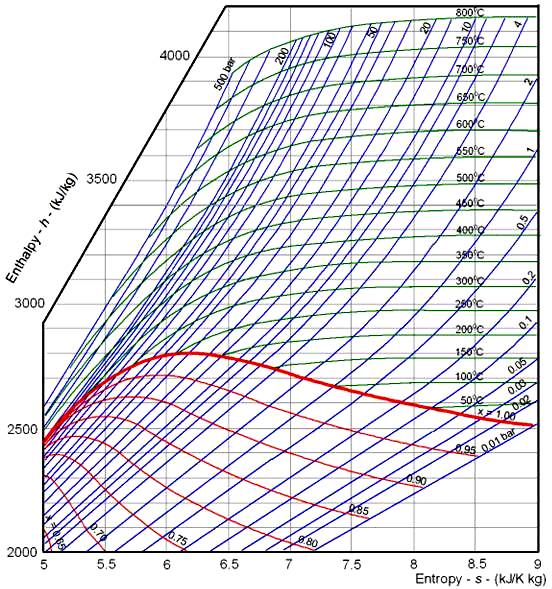
EnthalpyEntropy Diagram
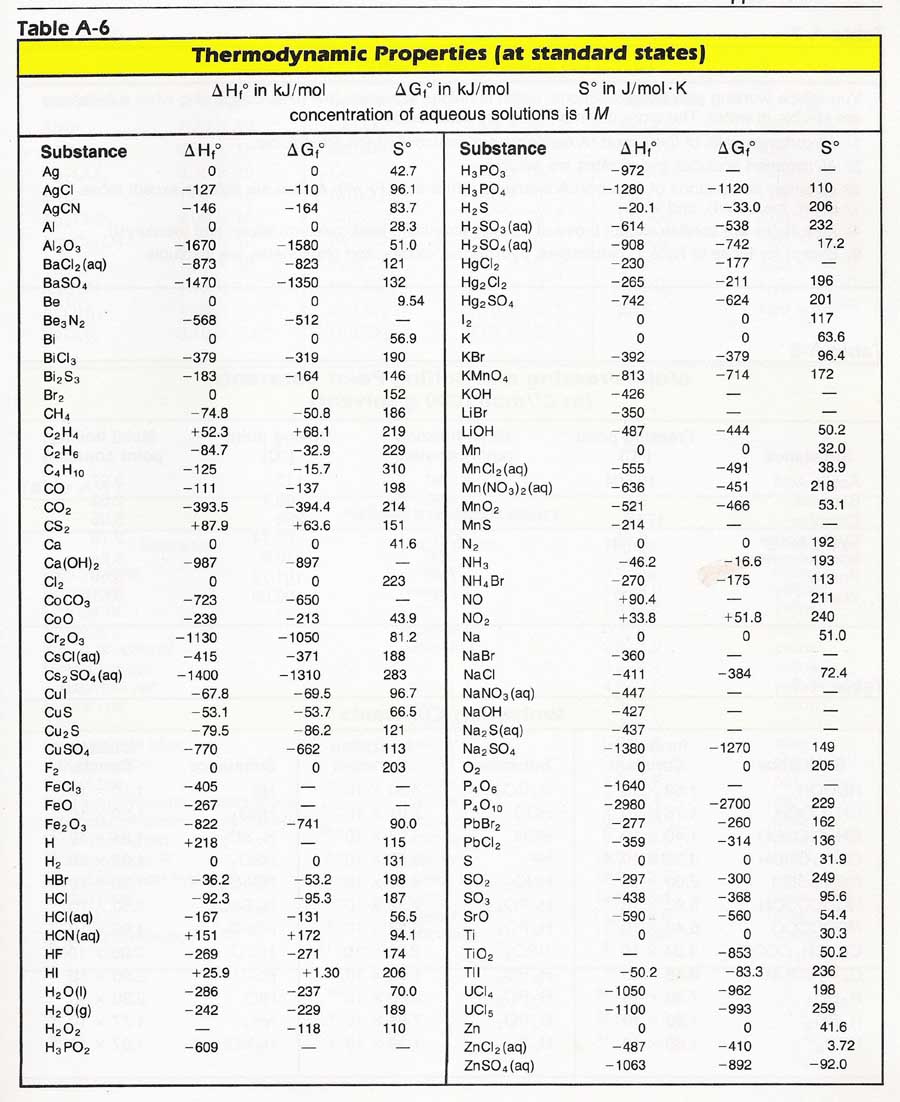
Entropy Table
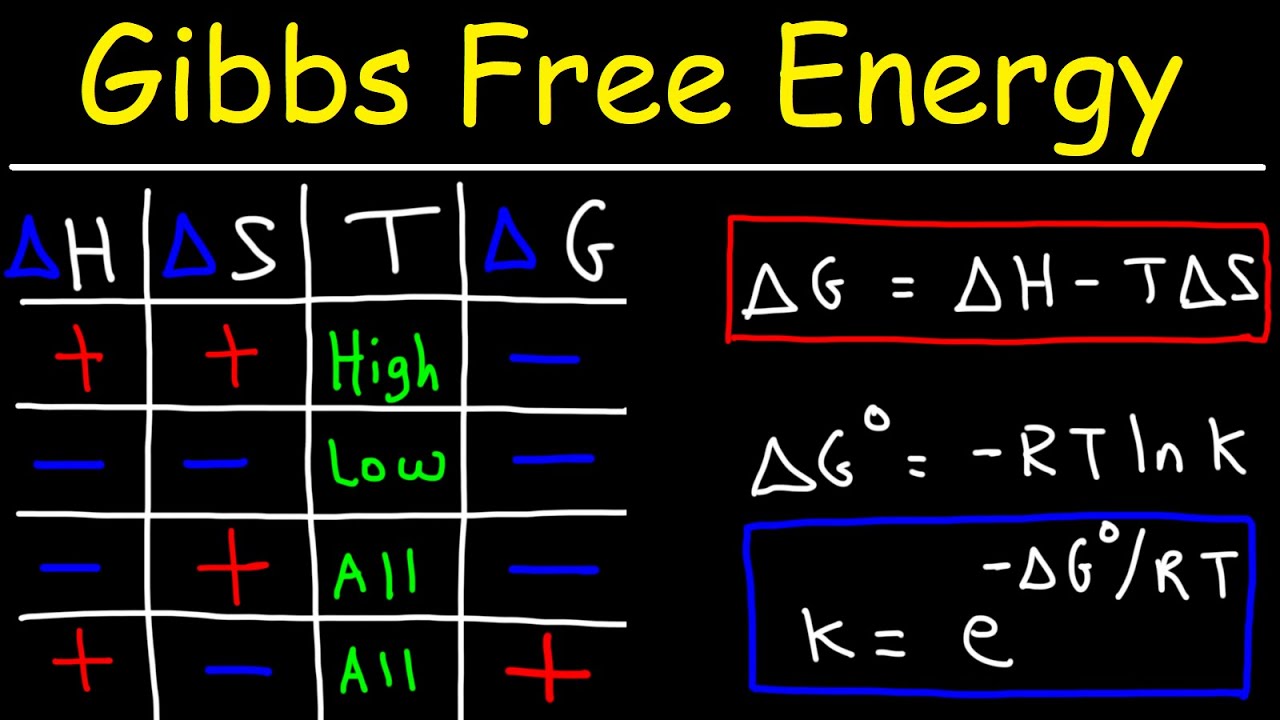
Gibbs Free Energy Entropy, Enthalpy & Equilibrium Constant K YouTube
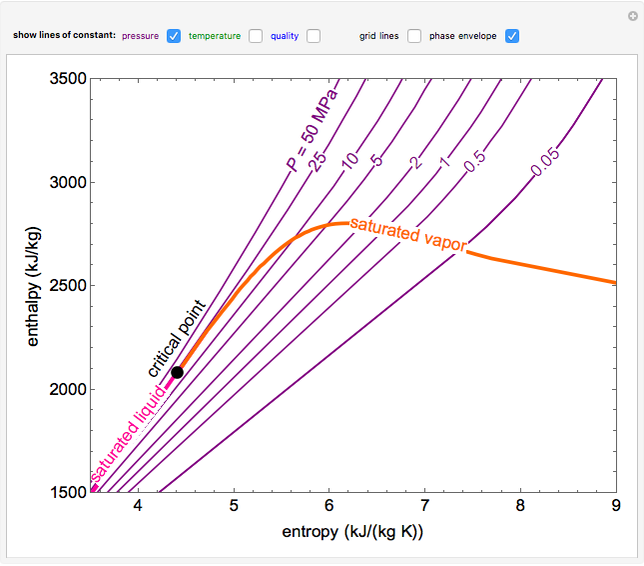
EnthalpyEntropy Diagram for Water Wolfram Demonstrations Project

EnthalpyEntropy Diagram for Water Wolfram Demonstrations Project
Enthalpy Chart
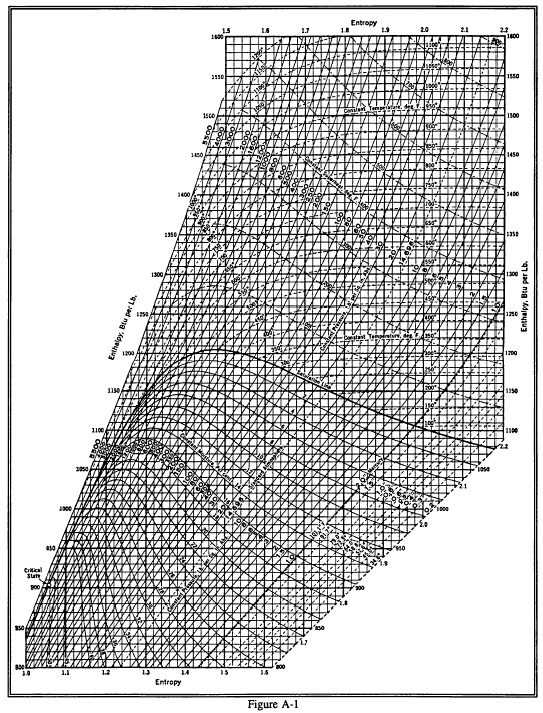
Enthalpy Entropy (hs) or Mollier Diagram Engineers Edge www
Web Enthalpy ( / ˈƐnθəlpi / Ⓘ) Is The Sum Of A Thermodynamic System 'S Internal Energy And The Product Of Its Pressure And Volume.
The Symbol For Enthalpy Is “H.” Enthalpy Is Also Considered To Be The Sum Of Internal Energy “U” And Flow Energy (Or Flow Work) P.v.
It Is A State Function In Thermodynamics Used In Many Measurements In Chemical, Biological, And Physical Systems At A Constant External Pressure, Which Is Conveniently Provided By The Large Ambient Atmosphere.
What Is The Enthalpy Change?
Related Post:
