Effective Nuclear Charge Chart
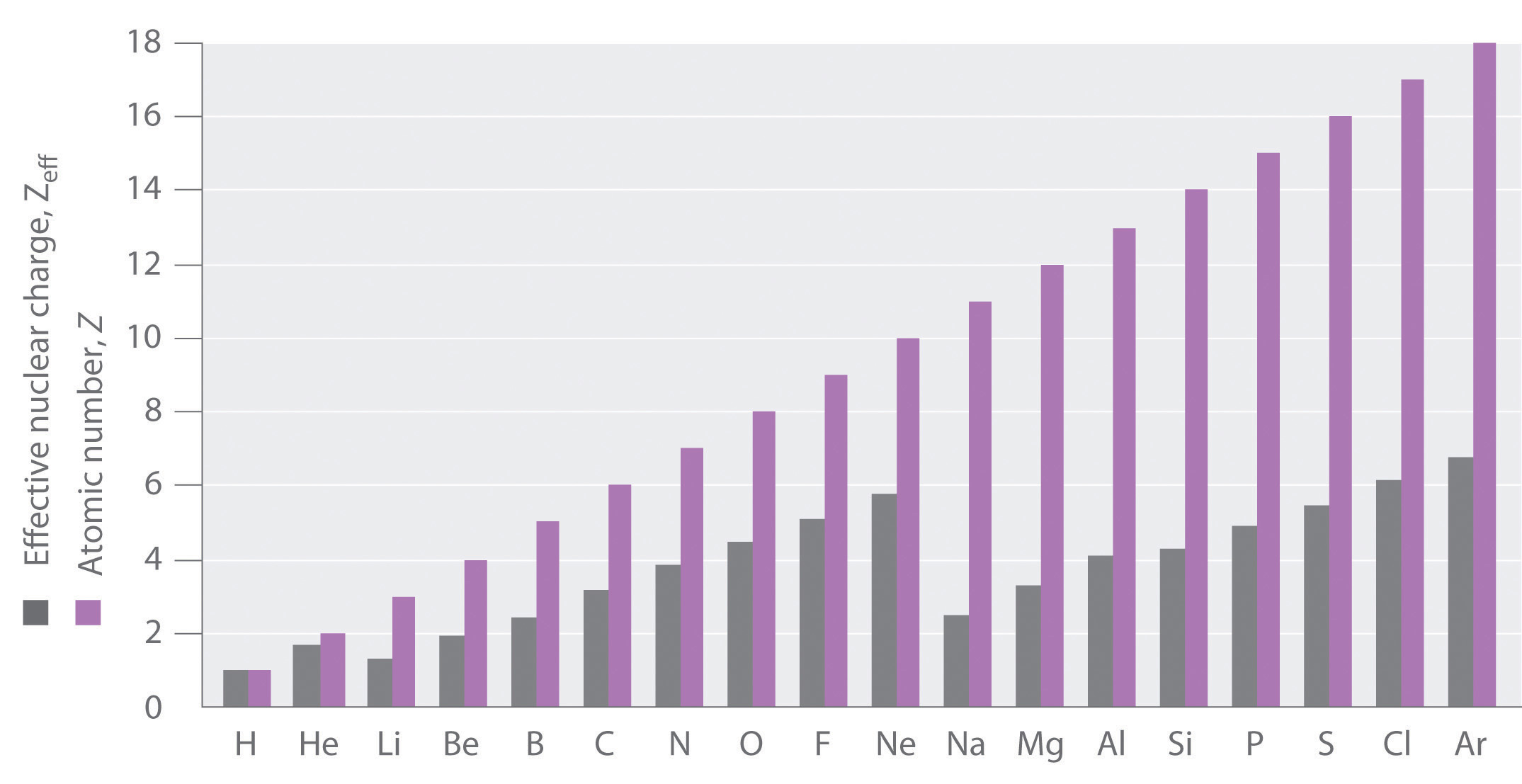
Effective Nuclear Charge Chart - Web the effective nuclear charge on an electron is given by the following equation: In a lithium atom, the nuclear charge (z) is +3. S = average amount of density between the nucleus and the electron. As will be shown, for example, as encø size × and as enc× size ø. 1 ≤ z eff ≤ z. At intermediate values of r, the effective nuclear charge is somewhere between 1 and z: Also, we solve this to find the effective charge of the electron. Fluorine has a high first ionization energy because it has such a high effective nuclear charge. Web more protons mean a greater effective nuclear charge, or a greater attractive force felt by the atom’s electrons to the nucleus. Web the two inner electrons in the 1 s orbital screen the third electron from the full effect of the nuclear +3 charge. Web the effective nuclear charge on an electron is given by the following equation: Zeff = the effective nuclear charge. Web the actual nuclear charge is the atomic number multiplied by the proton charge. Well first, oxygen has a higher first ionization energy compared to carbon. Ionization energy is the amount of energy needed to remove an electron from a. In a lithium atom, the nuclear charge (z) is +3. Web the actual nuclear charge is the atomic number multiplied by the proton charge. The difference between the full nuclear charge, z, and the screening effect of the inner two electrons is called the effective nuclear charge, or zeff. Electrons in s orbitals, even 4s or 5s, still spend some. At intermediate values of r, the effective nuclear charge is somewhere between 1 and z: Z = denotes the number of protons existing in the nucleus. Also, we solve this to find the effective charge of the electron. Notice that zeff = z only for hydrogen (figure 7.2.2 ). 1 ≤ z eff ≤ z. Web in chemistry, physics and materials science, the effective nuclear charge is a quantity used to predict the chemical behavior of atoms and molecules. Web the amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). At intermediate values of r, the effective nuclear charge is somewhere between 1 and z: 1 ≤ z. Web learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. Fluorine has a high first ionization energy because it has such a high effective nuclear charge. Web at r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or z eff ≈ z. Oxygen’s first ionization energy. The effective nuclear charge is always less than the actual nuclear charge [3]. Web the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly effective at shielding electrons in outer shells from the nuclear charge. Web in chemistry, physics and materials science, the. As will be shown, for example, as encø size × and as enc× size ø. So if we were to add an electron to a neutral atom of neon, neon has 10 protons, and 10 core electrons, so zeff = 10. Oxygen’s first ionization energy is 1313.9 kj/mol, while carbon is 1086.5 kj/mol. Web the amount of positive charge experienced. Web at r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or z eff ≈ z. Oxygen’s first ionization energy is 1313.9 kj/mol, while carbon is 1086.5 kj/mol. Web effective nuclear charge depends on the type of electron. Where z is the number of protons in the nucleus (atomic number), and s is. If this attractive force is greater, then the electrons are brought in closer to the nucleus resulting in a smaller atom. Web the effective nuclear charge on an electron is given by the following equation: Web so slater's rules help calculate the effective nuclear charge which quantifies the attraction an electron feels for an atom's nucleus. A similar trend can. Oxygen’s first ionization energy is 1313.9 kj/mol, while carbon is 1086.5 kj/mol. For example, in lithium (li), none of the three electrons feel the full +3 charge from the nucleus (see cartoon). The effective nuclear charge is always less than the actual nuclear charge [3]. 1 ≤ z eff ≤ z. The difference between the full nuclear charge, z, and. Also, we solve this to find the effective charge of the electron. Oxygen’s first ionization energy is 1313.9 kj/mol, while carbon is 1086.5 kj/mol. The difference between the full nuclear charge, z, and the screening effect of the inner two electrons is called the effective nuclear charge, or zeff. At intermediate values of r, the effective nuclear charge is somewhere between 1 and z: 1 ≤ zeff ≤ z. Web the amount of positive charge experienced by any individual electron is the effective nuclear charge (\(z_{eff}\)). Web in chemistry, physics and materials science, the effective nuclear charge is a quantity used to predict the chemical behavior of atoms and molecules. On the other hand, the effective nuclear charge is the net charge on the nucleus that attracts the valence electrons towards itself. Ionization energy is the amount of energy needed to remove an electron from a neutral gaseous atom and form an ion. Web describe the definition, formula, calculation, periodic table trend, and chart of effective nuclear charge. Web at r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. Where z is the number of protons in the nucleus (atomic number), and s is the number of electrons between the nucleus and the electron in question (the number of. 1s electrons experience an effective nuclear charge (z*) of +2.69, and 2s electrons experience an z* of +1.28. Z = denotes the number of protons existing in the nucleus. Web learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. Web more protons mean a greater effective nuclear charge, or a greater attractive force felt by the atom’s electrons to the nucleus.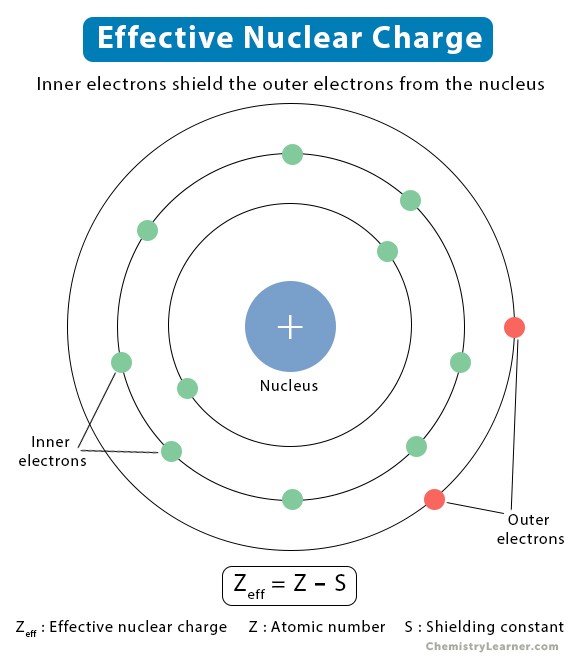
Effective Nuclear Charge Definition, Formula, and Chart

Nuclear Charge

PPT The Modern Periodic Table PowerPoint Presentation, free download

Plot of the effective nuclear charge values of 103 elements of the

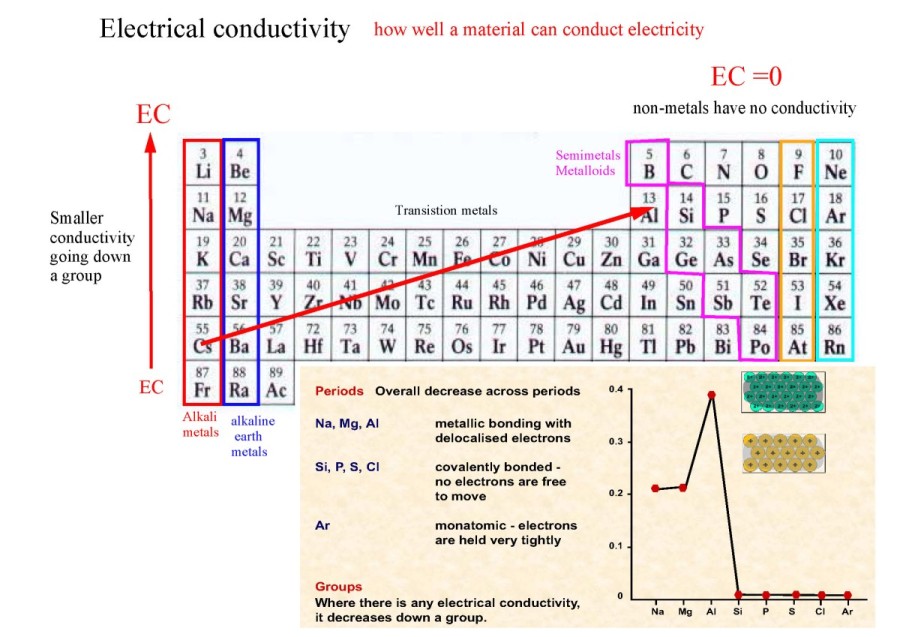
period 3 effective nuclear charge trend Best Online Free Chemistry
Periodic Table Trends Effective Nuclear Charge
7.2 Effective Nuclear Charge Chemistry LibreTexts

How To Find Nuclear Charge On Periodic Table

Comparative plot of the effective nuclear charge, electronegativity and
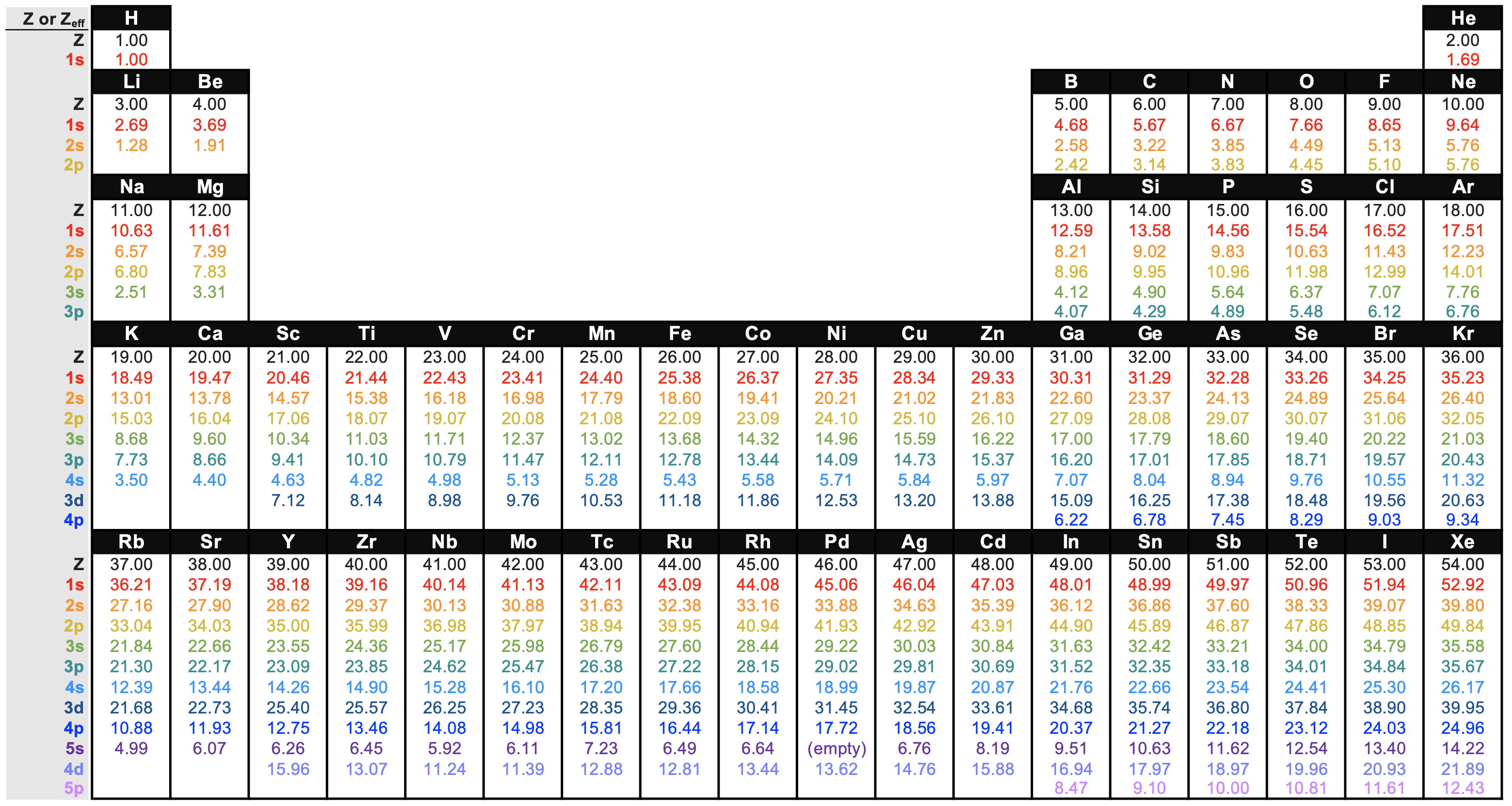
1.1.2 Effective Nuclear Charge Chemistry LibreTexts
If This Attractive Force Is Greater, Then The Electrons Are Brought In Closer To The Nucleus Resulting In A Smaller Atom.
At Intermediate Values Of R, The Effective Nuclear Charge Is Somewhere Between 1 And Z:
1 ≤ Z Eff ≤ Z.
For Example, In Lithium (Li), None Of The Three Electrons Feel The Full +3 Charge From The Nucleus (See Cartoon).
Related Post: