Dsur Template
Dsur Template - Web the development safety update report (dsur) proposed in this guideline is intended to be a common standard for periodic reporting on drugs under development (including marketed drugs that are under further study) among the ich regions. Web on the development safety update report (dsur): The report is submitted annually with a standardized format, enhancing communication between sponsors and regulators. Web a dsur is a critical document assessing and managing safety risks of investigational drugs during development. Web the recommended content and format of a dsur and provides an outline of points to be considered in its preparation and submission. Web the development safety update report (dsur) proposed in this guideline is intended to be a common standard for periodic reporting on drugs under development (including marketed drugs that are under further study) among the ich regions. Web the development safety update report (dsur) proposed in this guideline is intended to be the common standard for annual clinical trial safety reporting among the ich regions and can be submitted instead of existing reports including the us ind annual report and the eu annual safety report. Harmonizing the format and content for periodic safety reporting during clinical trials. It defines the recommended content and format of a dsur and provides an outline of points to be considered in its preparation and submission. Web the development safety update report (dsur) proposed in this guidance is intended to be a common standard for periodic reporting on drugs under development (including marketed drugs that are. Web the recommended content and format of a dsur and provides an outline of points to be considered in its preparation and submission. Web a dsur is a critical document assessing and managing safety risks of investigational drugs during development. Web the development safety update report (dsur) proposed in this guideline is intended to be the common standard for annual. Web the development safety update report (dsur) proposed in this guideline is intended to be a common standard for periodic reporting on drugs under development (including marketed drugs that are under further study) among the ich regions. It defines the recommended content and format of a dsur and provides an outline of points to be considered in its preparation and. Web the development safety update report (dsur) proposed in this guideline is intended to be the common standard for annual clinical trial safety reporting among the ich regions and can be submitted instead of existing reports including the us ind annual report and the eu annual safety report. Web a dsur is a critical document assessing and managing safety risks. The report is submitted annually with a standardized format, enhancing communication between sponsors and regulators. Web the recommended content and format of a dsur and provides an outline of points to be considered in its preparation and submission. It defines the recommended content and format of a dsur and provides an outline of points to be considered in its preparation. Web the development safety update report (dsur) proposed in this guideline is intended to be a common standard for periodic reporting on drugs under development (including marketed drugs that are under further study) among the ich regions. It defines the recommended content and format of a dsur and provides an outline of points to be considered in its preparation and. Web on the development safety update report (dsur): Web the development safety update report (dsur) proposed in this guidance is intended to be a common standard for periodic reporting on drugs under development (including marketed drugs that are. The report is submitted annually with a standardized format, enhancing communication between sponsors and regulators. Harmonizing the format and content for periodic. Web the development safety update report (dsur) proposed in this guideline is intended to be a common standard for periodic reporting on drugs under development (including marketed drugs that are under further study) among the ich regions. Web the recommended content and format of a dsur and provides an outline of points to be considered in its preparation and submission.. Web the development safety update report (dsur) proposed in this guideline is intended to be a common standard for periodic reporting on drugs under development (including marketed drugs that are under further study) among the ich regions. Web the development safety update report (dsur) proposed in this guidance is intended to be a common standard for periodic reporting on drugs. Web a dsur is a critical document assessing and managing safety risks of investigational drugs during development. Web the development safety update report (dsur) proposed in this guideline is intended to be a common standard for periodic reporting on drugs under development (including marketed drugs that are under further study) among the ich regions. Web the development safety update report. It defines the recommended content and format of a dsur and provides an outline of points to be considered in its preparation and submission. Web the development safety update report (dsur) proposed in this guideline is intended to be the common standard for annual clinical trial safety reporting among the ich regions and can be submitted instead of existing reports. It defines the recommended content and format of a dsur and provides an outline of points to be considered in its preparation and submission. Web the development safety update report (dsur) proposed in this guideline is intended to be the common standard for annual clinical trial safety reporting among the ich regions and can be submitted instead of existing reports including the us ind annual report and the eu annual safety report. Web on the development safety update report (dsur): Web a dsur is a critical document assessing and managing safety risks of investigational drugs during development. Web the development safety update report (dsur) proposed in this guideline is intended to be a common standard for periodic reporting on drugs under development (including marketed drugs that are under further study) among the ich regions. Web the development safety update report (dsur) proposed in this guideline is intended to be a common standard for periodic reporting on drugs under development (including marketed drugs that are under further study) among the ich regions. The report is submitted annually with a standardized format, enhancing communication between sponsors and regulators. Web the recommended content and format of a dsur and provides an outline of points to be considered in its preparation and submission.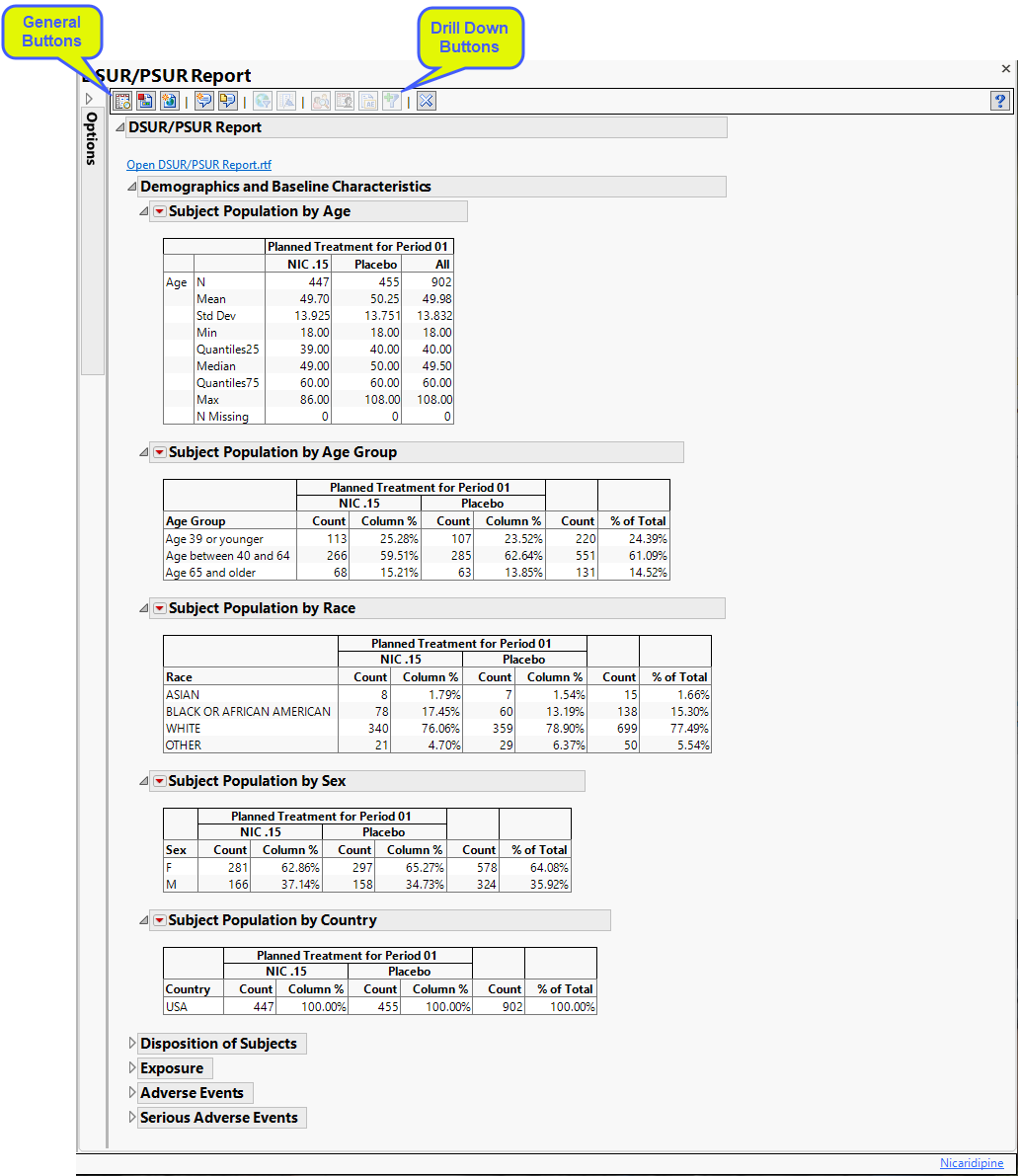
DSUR/PSUR Report
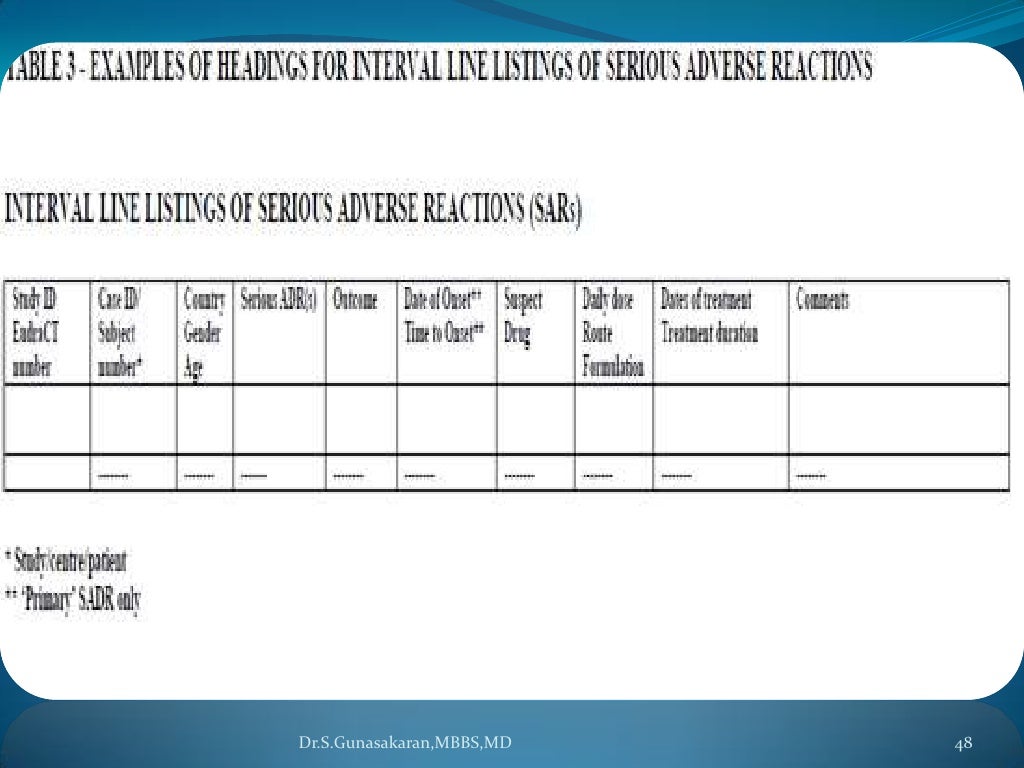
DSUR

Template for the Development Safety Update Report (DSUR) with

Development Safety Update Report (DSUR) Doc Template pdfFiller

Webinar PSUR under the Medical Device Regulation Practical Guide

Template for the Development Safety Update Report (DSUR) with

Development Safety Update Report (DSUR)
+(1).jpg)
Aspects of pharmacovigilance Development Safety Update Report (DSUR

Appendix 1 DSUR Template
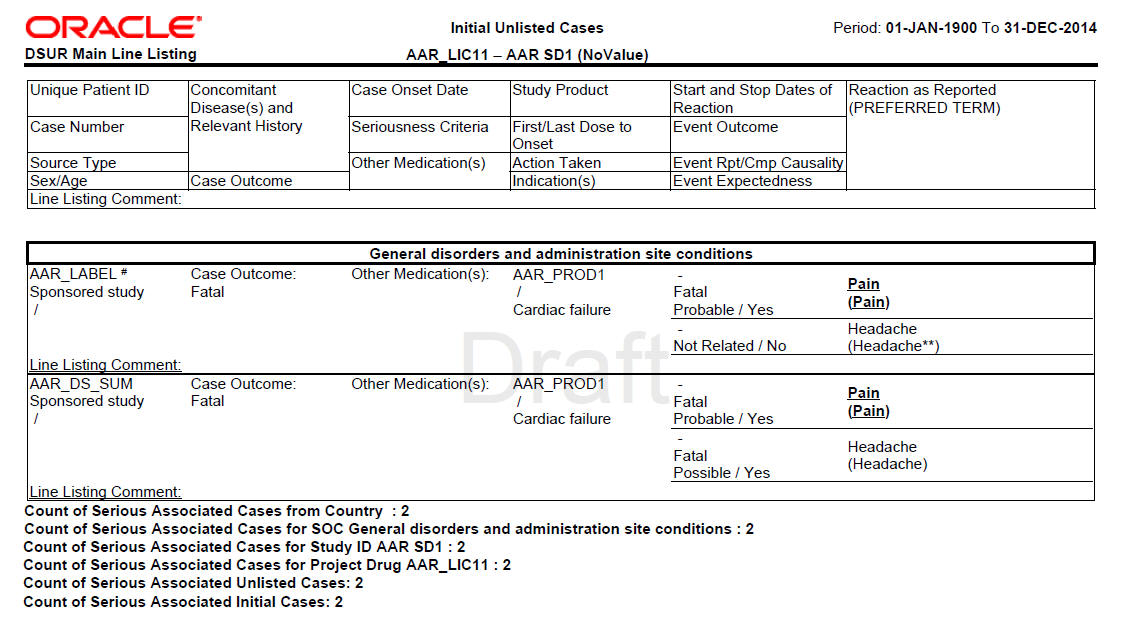
Development Safety Update Report
Web The Development Safety Update Report (Dsur) Proposed In This Guidance Is Intended To Be A Common Standard For Periodic Reporting On Drugs Under Development (Including Marketed Drugs That Are.
Harmonizing The Format And Content For Periodic Safety Reporting During Clinical Trials.
Related Post: