Drawing Of The Reaction Of Hydrochloric Acid With Water
Drawing Of The Reaction Of Hydrochloric Acid With Water - Web hydrochloric acid is a strong acid which dissociates into h + and cl − ions in water. The resulting hydrogen chloride gas is absorbed in deionized water, resulting in chemically. Web the production of more than 90% of all chemical products we use in our everyday lives relies on catalysts. In this reaction, a proton is transferred from hcl. Includes kit list and safety instructions. In reality, this reaction reaches an equilibrium. (i) draw a diagram to show the arrangement used for the absorption of hcl gas. The reaction takes place as follows: While the concentration of the acid may vary, a 15% hydrochloric acid mix is. Draw a cross on a piece of paper. Web the production of more than 90% of all chemical products we use in our everyday lives relies on catalysts. Web hcl(aq) hx+(aq) +clx−(aq) h c l ( a q) h x + ( a q) + c l x − ( a q) i understand that when added to water the h h leaves its electron to the cl. Web write a balanced chemical equation and state symbols for the reaction: In reality, this reaction reaches an equilibrium. (i) draw a diagram to show the arrangement used for the absorption of hcl gas. Measure 5 cm 3 of dilute hydrochloric acid into a measuring cylinder. Web for example, hydrochloric acid, hcl, as a strong acid it donates a proton. Web there are tables of electrode reactions, descriptions of experimental methods of electrolysis and a summary table of the electrolysis products from many common melts. Since the h+ (often called a. Web hcl(aq) hx+(aq) +clx−(aq) h c l ( a q) h x + ( a q) + c l x − ( a q) i understand that when added. Web the reactions of hydrochloric acid are those of typical strong acids, such as: While the concentration of the acid may vary, a 15% hydrochloric acid mix is. This process is a highly exothermic reaction. The resulting hydrogen chloride gas is absorbed in deionized water, resulting in chemically. Measure 5 cm 3 of dilute hydrochloric acid into a measuring cylinder. In reality, this reaction reaches an equilibrium. Measure 50 cm 3 of sodium thiosulfate solution into a flask. The resulting hydrogen chloride gas is absorbed in deionized water, resulting in chemically. This process is a highly exothermic reaction. In this reaction, a proton is transferred from hcl. Web hydrochloric acid is the single largest liquid component used in a fracturing fluid aside from water; Sodium hydroxide solution (in water) reacts with hydrochloric acid solution (in water) to produce sodium. If you paired water with something which is a weaker acid than water, then water will act as an acid. Write reactions of acids with metals. Web in. In this reaction, a proton is transferred from hcl. Web hcl(aq) hx+(aq) +clx−(aq) h c l ( a q) h x + ( a q) + c l x − ( a q) i understand that when added to water the h h leaves its electron to the cl c l atom. Web hydrochloric acid is a strong acid, stronger. Includes kit list and safety instructions. Reactions with metals in which hydrogen gas is displaced, reactions with basic (metal). Measure 50 cm 3 of sodium thiosulfate solution into a flask. (i) draw a diagram to show the arrangement used for the absorption of hcl gas. Measure 5 cm 3 of dilute hydrochloric acid into a measuring cylinder. Write reactions of acids with metals. Web there are tables of electrode reactions, descriptions of experimental methods of electrolysis and a summary table of the electrolysis products from many common melts. In this reaction, a proton is transferred from hcl. While the concentration of the acid may vary, a 15% hydrochloric acid mix is. Sodium hydroxide solution (in water) reacts. Since the h+ (often called a. Measure 50 cm 3 of sodium thiosulfate solution into a flask. This process is a highly exothermic reaction. The resulting hydrogen chloride gas is absorbed in deionized water, resulting in chemically. Includes kit list and safety instructions. Web write a balanced chemical equation and state symbols for the reaction: In this reaction, a proton is transferred from hcl. (i) draw a diagram to show the arrangement used for the absorption of hcl gas. If you paired water with something which is a weaker acid than water, then water will act as an acid. Write reactions of bases with metals. This process is a highly exothermic reaction. Web the reaction of calcium carbonate and hydrochloric acid. While the concentration of the acid may vary, a 15% hydrochloric acid mix is. Web in the laboratory preparation of hydrochloric acid, hydrogen chloride gas is dissolved in water. Sodium hydroxide solution (in water) reacts with hydrochloric acid solution (in water) to produce sodium. Reactions with metals in which hydrogen gas is displaced, reactions with basic (metal). Web the production of more than 90% of all chemical products we use in our everyday lives relies on catalysts. Web in this video we will look at the equation for hcl + h2o and write the products. Web investigate the effects of changing the conditions of a reaction on the rates of chemical reactions by measuring the production of a gas (in the reaction between hydrochloric. In reality, this reaction reaches an equilibrium. Since the h+ (often called a.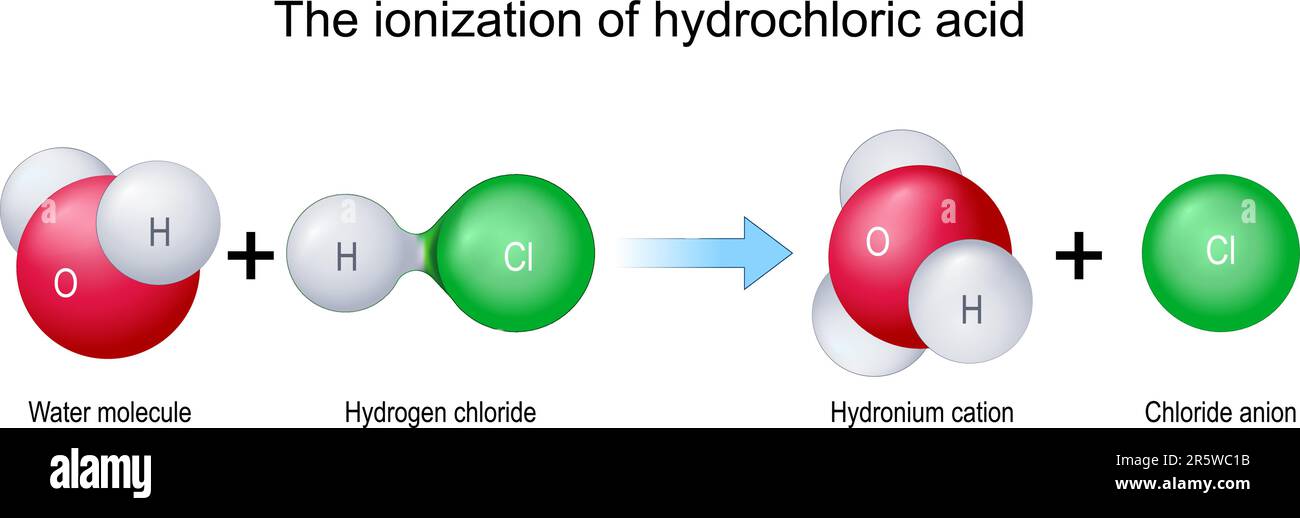
The ionization of hydrochloric acid. Molecules H2O and HCl combine to
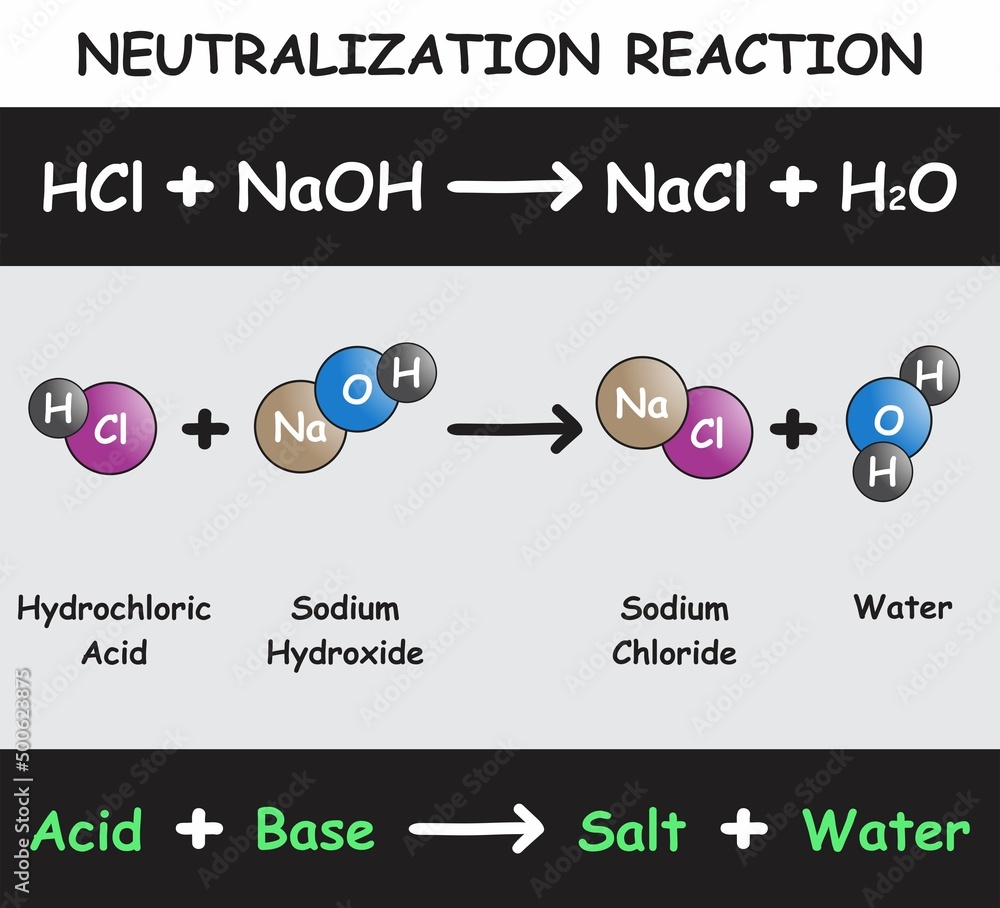
Neutralization Reaction Infographic Diagram with example of
What is the chemical equation for HCl dissolving into water and

Reaction of Hydrochloric Acid with Water, Chemistry Lecture Sabaq.pk
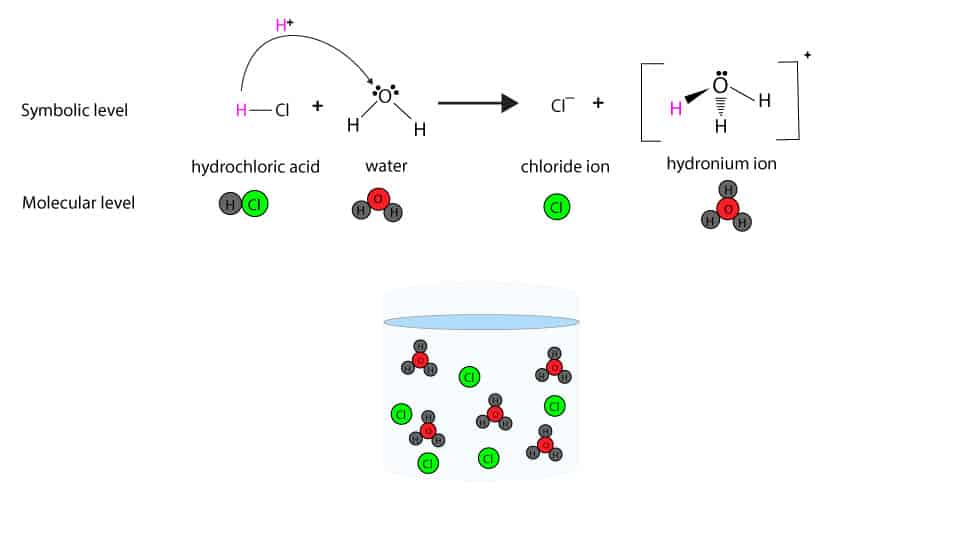
Chemical Equation For The Acid Ionization Of Hydrochloric Hcl In Water
Chemical Formula For Hydrochloric Acid
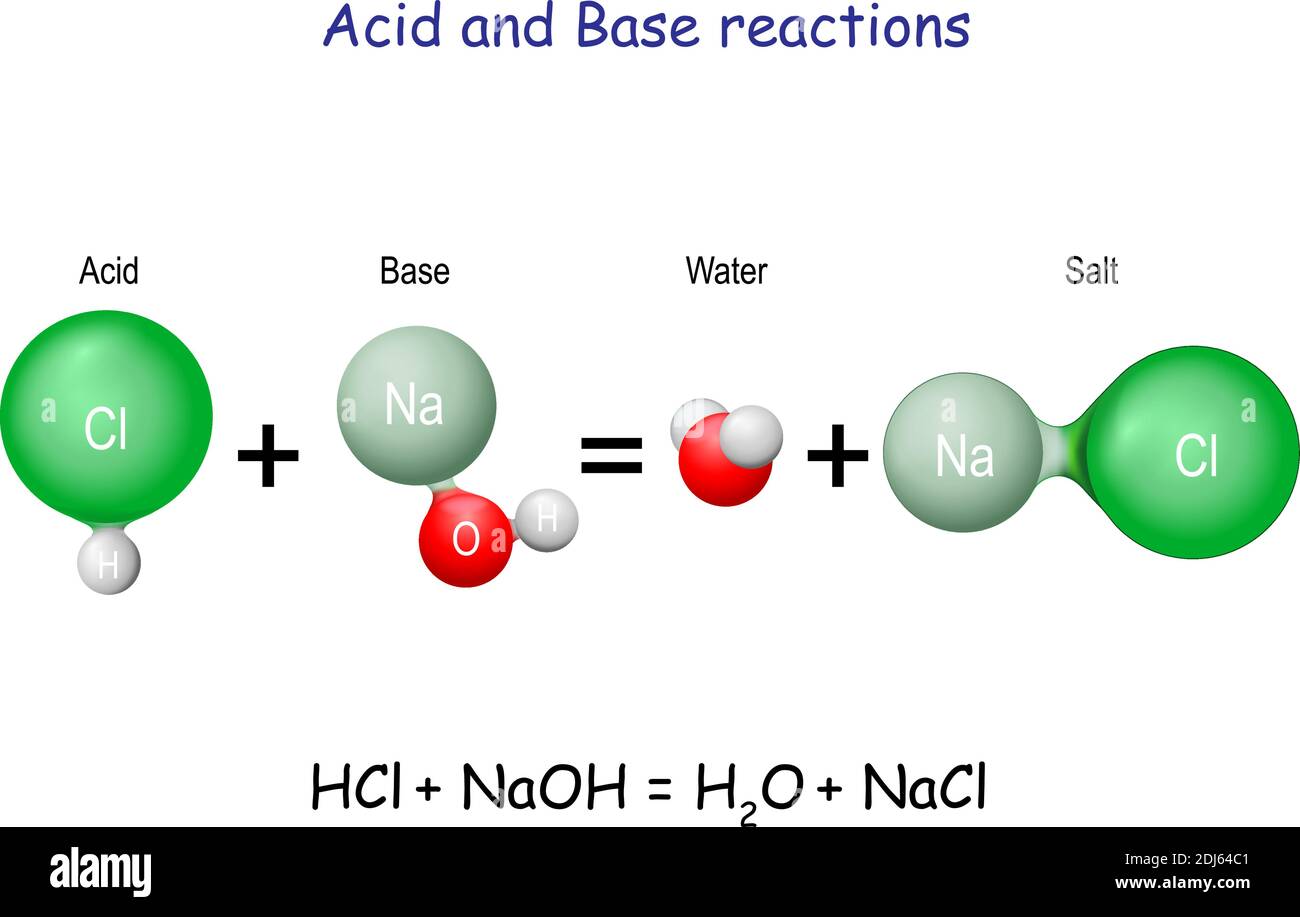
Hydrochloric acid molecule Stock Vector Images Alamy

6.3 AcidBase Reactions CHEM 1114 Introduction to Chemistry

Electrolysis of Water & Hydrochloric Acid Reactions Chemistry
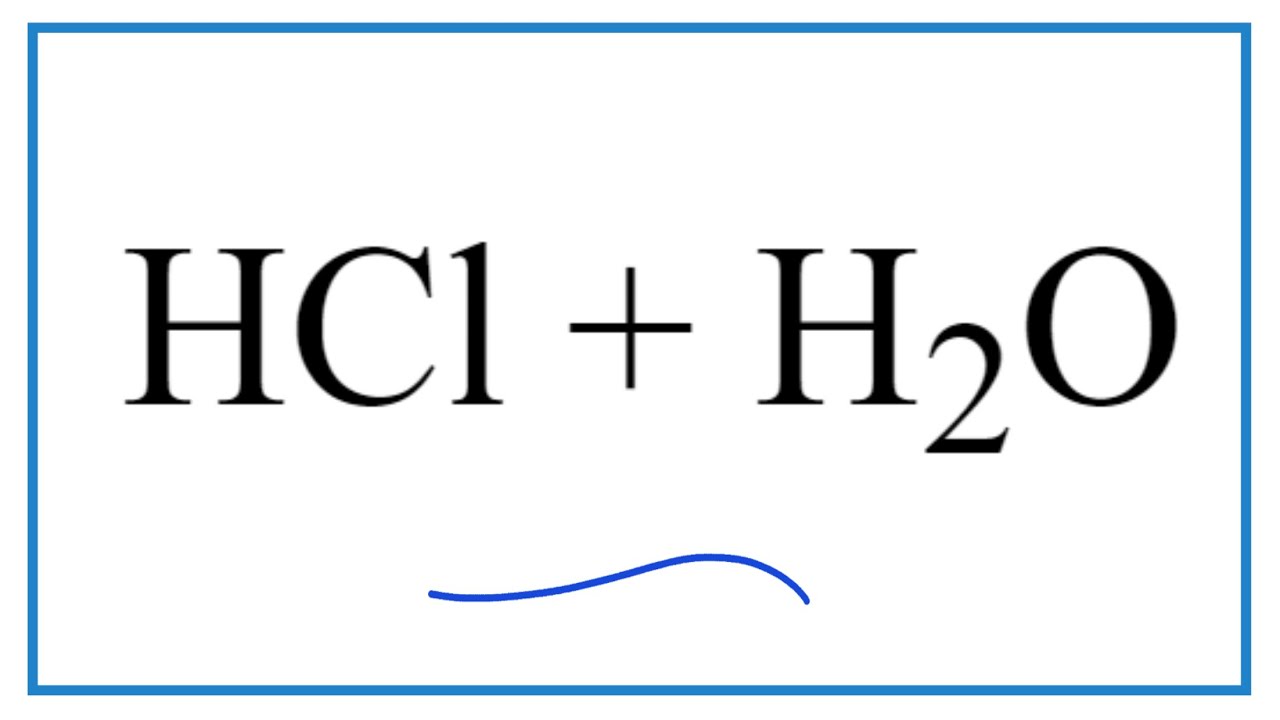
Complete The Chemical Equation For Acid Ionization Of Hydrochloric Hcl
Web Hydrochloric Acid Is A Strong Acid Which Dissociates Into H + And Cl − Ions In Water.
Web As The Reaction Is Exothermic, The Installation Is Called An Hcl Oven Or Hcl Burner.
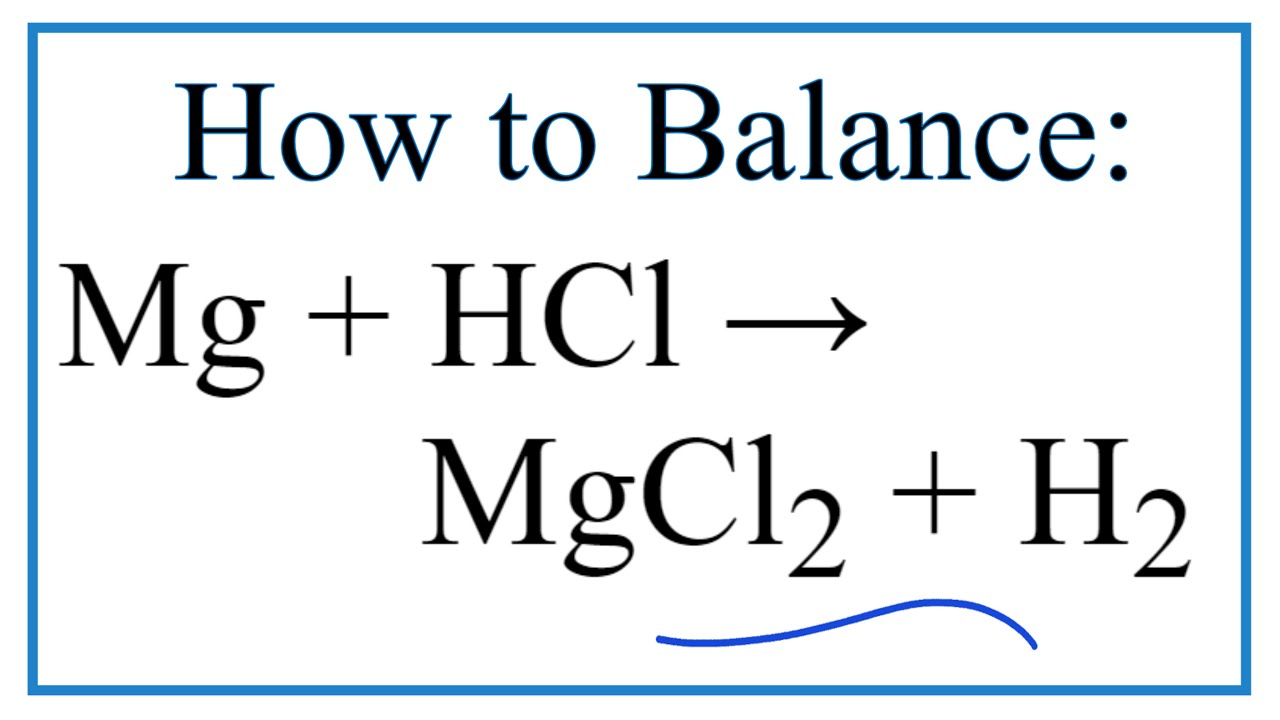
Web A Class Practical On Reacting Magnesium With Hydrochloric Acid And How To Measure The Rate Of Reaction.
Web Hcl(Aq) Hx+(Aq) +Clx−(Aq) H C L ( A Q) H X + ( A Q) + C L X − ( A Q) I Understand That When Added To Water The H H Leaves Its Electron To The Cl C L Atom.
Related Post: