Drawing Of Charles Law
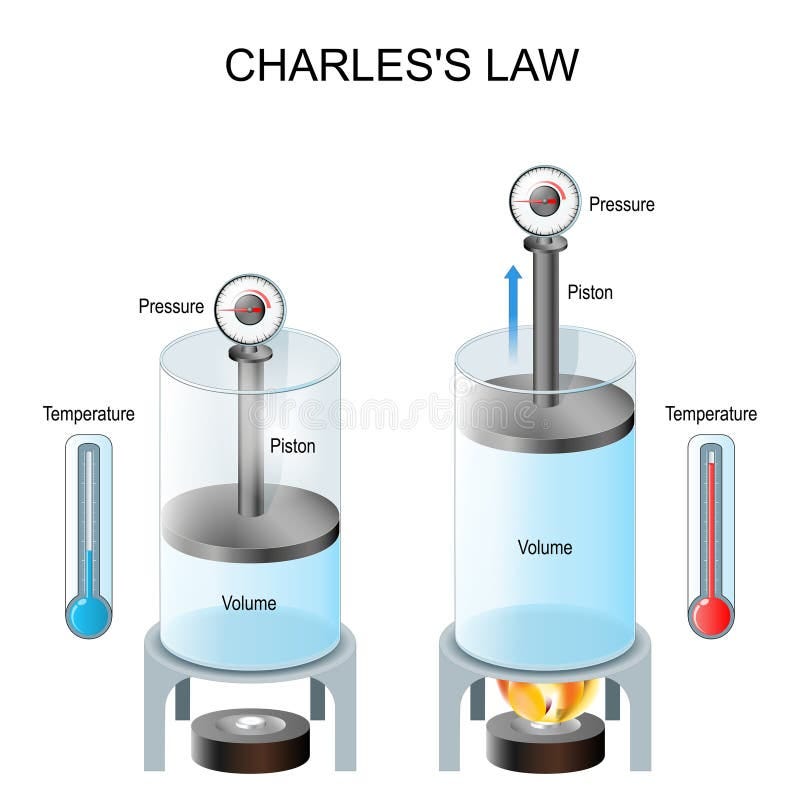
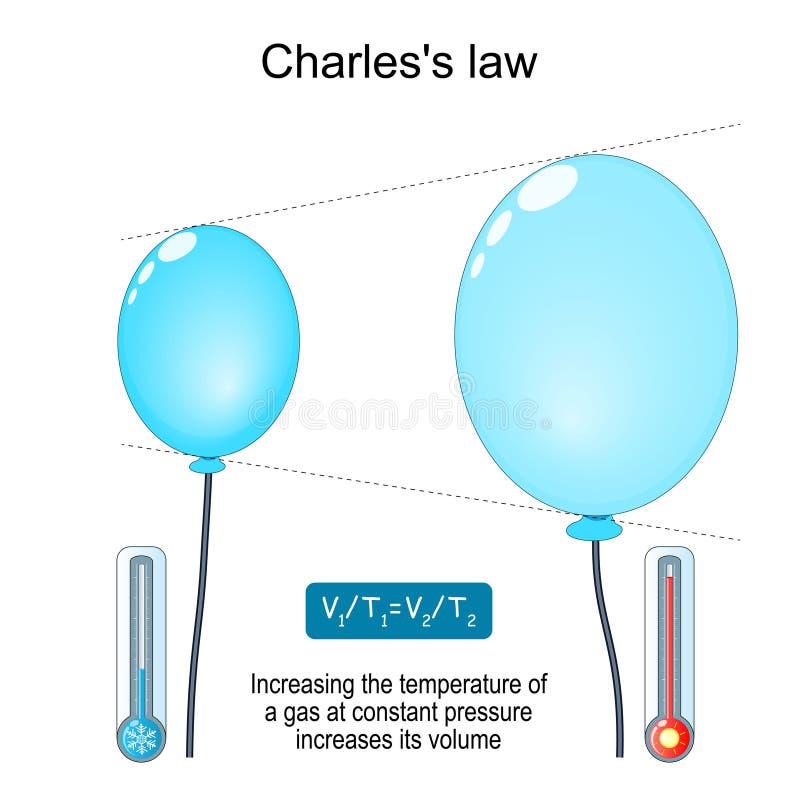
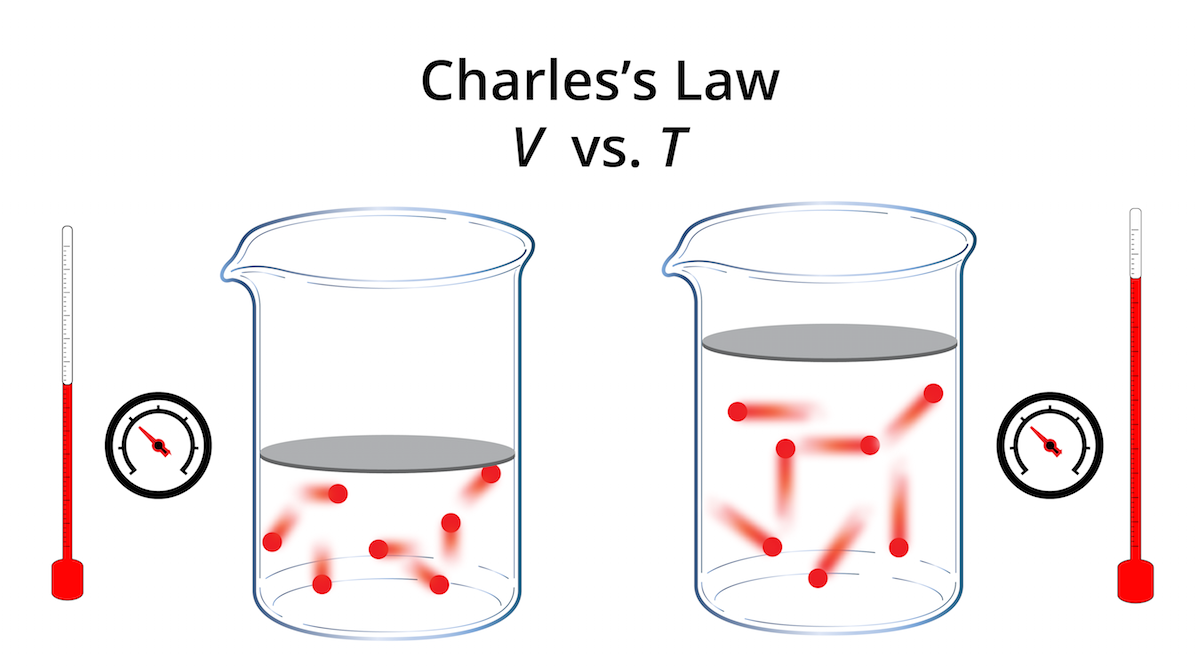
Drawing Of Charles Law - At constant pressure the volume of a given mass of a gas increases or decreases by 1/273 of its volume at 0 o c for every degree rise or fall in temperature. Doubling the temperature of a gas doubles its volume. The volume (v) of a confined gas (n) is directly proportional to its absolute temperature (t), provided its pressure (p) remains constant. Web the behavior of gases can be modeled with gas laws. The law suggests that the volume of a gas is directly proportional to its temperature when the pressure is kept constant. Web boyle’s law states the relation between volume and pressure at constant temperature and mass. When we compare the substance under initial ( v₁, t₁) and final conditions ( v₂, t₂ ), you can write charles' law as v₁/t₁ = v₂/t₂. So, as the temperature decreases the particles have occupy a smaller volume if the pressure is to remain constant. In gas laws, temperatures must always be expressed in kelvins. Charles's law states that the volume of an ideal gas changes proportionally to the temperature of that gas, given that pressure and amount of gas present are held constant. It states that under a constant temperature when the pressure on a gas increases its volume decreases. Charles's law states that the volume of an ideal gas changes proportionally to the temperature of that gas, given that pressure and amount of gas present are held constant. It gives a formal relationship between temperature and volume. There are four laws, known. This law describes how gases will expand when heated. The pressure is caused by the gas molecules bumping into the walls of the container; At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in kelvins). Boyle showed that the volume of a sample of a gas is inversely. Charles's law relates. Web charles' law examines the relationship between the volume of a gas and its temperature. Doubling the temperature of a gas doubles its volume. So you would do an experiment in which you measure the volume of a gas at various temperatures. Web charles’s law or the law of volumes is an ideal gas law that states that the volume. Find more details like charles law formula, derivation, and application on this page. The temperature must be measured with the kelvin scale. There are four laws, known as gas laws, which describe how gases behave. Web charles' law was formulated by the famous scientist jacques charles, less than 2 centuries after boyle’s law, in 1800. Web charle’s law states that. Web the behavior of gases can be modeled with gas laws. The absolute temperature is temperature measured with the kelvin scale. Web charles’s law or the law of volumes is an ideal gas law that states that the volume and temperature of a fixed amount of gas are proportional at constant pressure. Charles's law relates a gas's volume and temperature. Web charles' law was formulated by the famous scientist jacques charles, less than 2 centuries after boyle’s law, in 1800. Web charles' law examines the relationship between the volume of a gas and its temperature. The law suggests that the volume of a gas is directly proportional to its temperature when the pressure is kept constant. Web boyle’s law states. The equation for charles's law can be expressed as v 1 /t 1 =v 2 /t 2. The relationship between the volume of a gas and temperature was observed by jacques charles in 1787. This relationship of direct proportion can be. When the pressure on a sample of a dry gas is held constant, the kelvin temperature and the volume. Therefore, v ∝ t or, v = kt, p &. The volume of a gas increases with an increase in temperature at constant pressure and vice versa. Robert boyle conducted an experiment on gases to study the deviation of its behaviour in changed physical conditions. Web charles' law was formulated by the famous scientist jacques charles, less than 2 centuries. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in kelvins). Web charles’s law implies that the volume of a gas is directly proportional to its absolute temperature. At constant pressure, the volume of a given mass of an ideal gas increases or decreases by the same factor as its temperature. At constant pressure, the volume of a given mass of an ideal gas increases or decreases by the same factor as its temperature on the absolute temperature scale (i.e. Web charles law is an ideal gas law that establishes a relation between volume and temperature at constant pressure. There’s no duo that takes method dressing more seriously than zendaya and. This law describes how gases will expand when heated. The relationship between the volume of a gas and temperature was observed by jacques charles in 1787. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in kelvins). Web charles' law, also known as the law of volumes, describes how gases tend to expand when heated. Web charles law is an ideal gas law that establishes a relation between volume and temperature at constant pressure. Robert boyle conducted an experiment on gases to study the deviation of its behaviour in changed physical conditions. Let’s assume you get the following data. Web how to demonstrate charles's law. It can be stated as: Charles's law relates a gas's volume and temperature at constant pressure and amount. The absolute temperature is temperature measured with the kelvin scale. There’s no duo that takes method dressing more seriously than zendaya and law roach. The temperature must be measured with the kelvin scale. This relationship, illustrated in part (b) in figure \(\pageindex{3}\) is often referred to as charles’s law and is stated mathematically as The volume of a gas increases with an increase in temperature at constant pressure and vice versa. Web charles’ law can be stated as follows: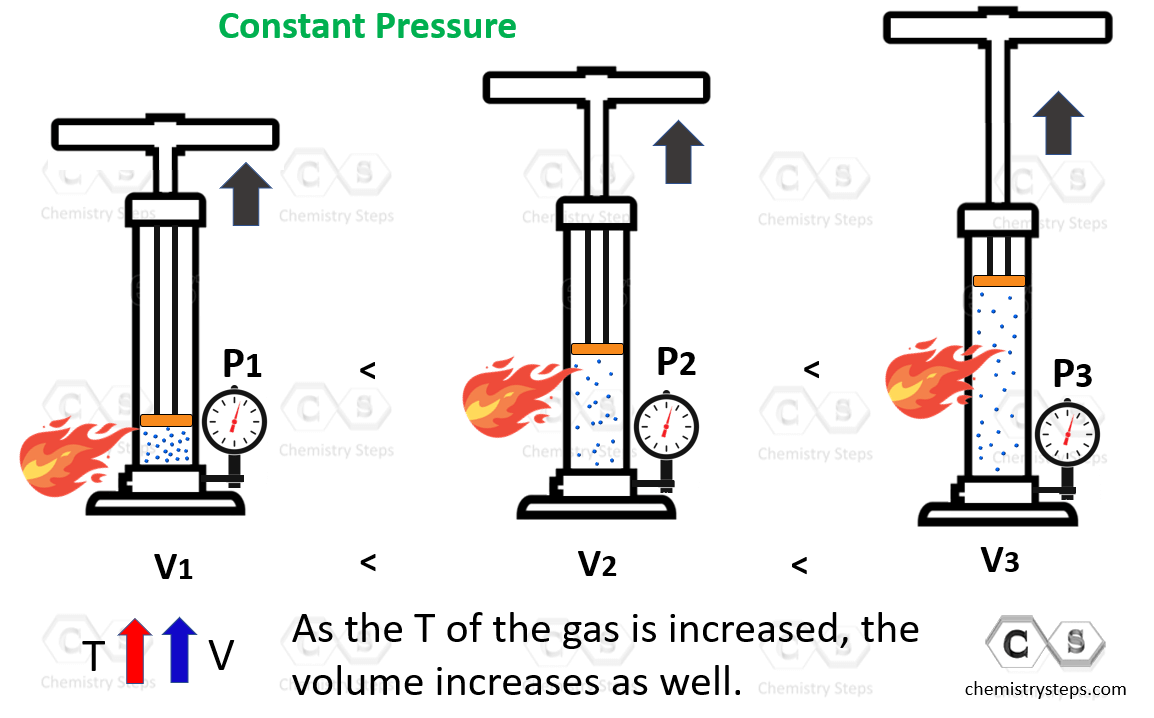
Charle’s Law Chemistry Steps
Charles law volume and temperature Teach Chemistry

Charles’ Law Statement, Formula, Examples, and Graph

Charles's Law Definition, Formula, Examples
Charles S Law. Relationship between Volume and Temperature Stock Vector
Properties of Gases Chemistry Visionlearning
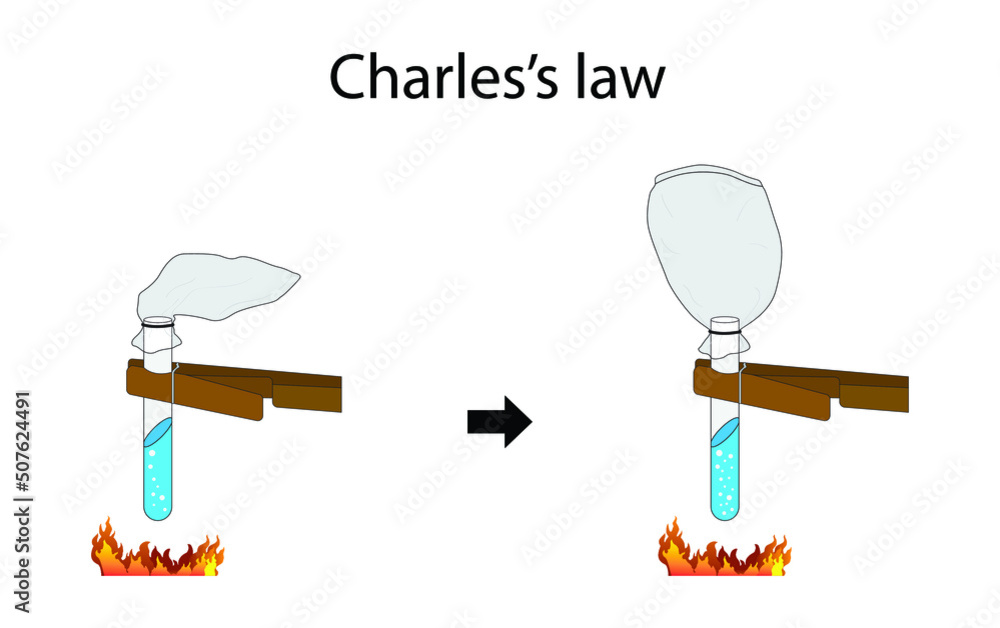
illustration of physics and chemistry, Charles' law is an experimental

Chemistry Charles's Law (Gas Laws) with 2 examples Charles law

Charless Law Relationship Between Volume Temperature Stock Vector
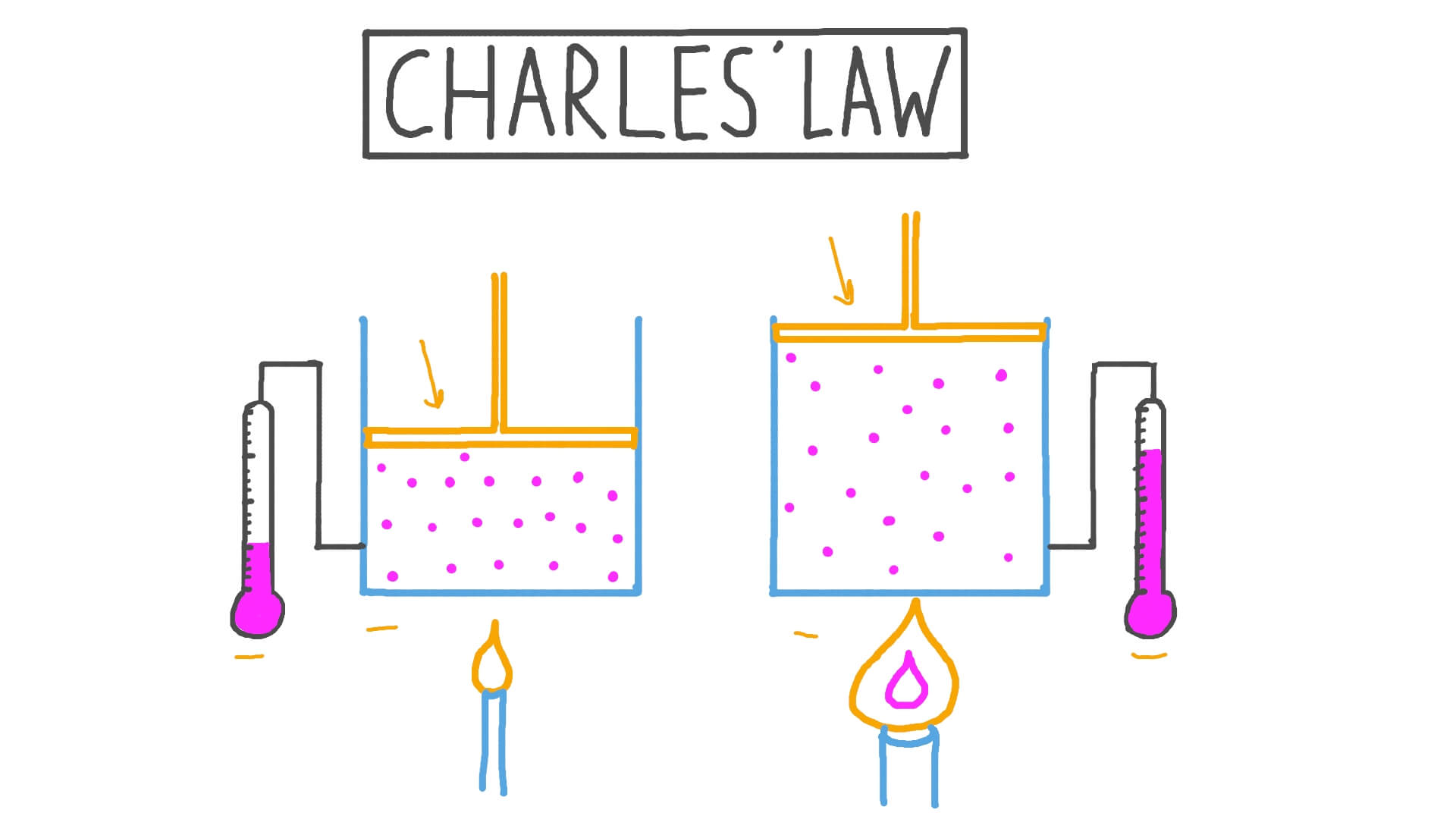
Unit 1 Behavior of Matter & Properties of Gases
Web Charles' Law Describes The Relationship Between The Volume And Temperature Of A Fixed Mass Of Gas That Is Held At A Fixed Pressure.
It Was First Stated By French Scientist Jacques Charles In 1787.
Web Charles’s Law Implies That The Volume Of A Gas Is Directly Proportional To Its Absolute Temperature.
Boyle Showed That The Volume Of A Sample Of A Gas Is Inversely.
Related Post: