Drawing Of Carbon Atom
Drawing Of Carbon Atom - Let’s draw and understand this lewis dot structure step by step. Web this image can be made accessible with an image description, by creating a tactile graphic, or by printing a 3d object of the image. Web steps of drawing the lewis structure of co 2 are explained in detail in this tutorial. It is nonmetallic and tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. Web the small grey dot that follows your cursor is an atom (carbon). And each oxygen is six, and we have one of them, so six. In this toolbar you can select from a number of elements, you can also pick an element from the periodic table using the last button. This video shows how to use the periodic table to draw lewis structures and figure out how many valence electrons an atom has. Then play a game to test your ideas! During this course, you will view molecules written in all three forms. Web the small grey dot that follows your cursor is an atom (carbon). Then play a game to test your ideas! Each hydrogen is one valence electron, but we have two of them, so 1 times 2. Web in carbon dioxide, the carbon atom has double bonds to oxygen on both sides (o=c=o). As long as it’s carbon it has. Then play a game to test your ideas! The carbon atom does not have a lone pair while both the oxygen atoms have 2 lone pairs. Later on in this chapter and throughout this book we will see examples of organic ions called ‘carbocations’ and carbanions’, in which a carbon atom bears a positive or negative formal charge, respectively. Web. Two oxygen atoms are found at the terminals, where they share electrons and form a double bond with the carbon atom. It belongs to group 14 of the periodic table. In this toolbar you can select from a number of elements, you can also pick an element from the periodic table using the last button. Then play a game to. You can simplify the formula by writing, for example, ch 3 or ch 2 instead of showing all these bonds. The carbon atom does not have a lone pair while both the oxygen atoms have 2 lone pairs. The choice of modality varies depending on factors such as the information to be conveyed, grade level, student knowledge and experience, and. In the first step, we will draw the nucleus of the carbon atom. Carbon makes up about 0.025 percent of earth's crust. The circle with the c is a representative nucleus, so now you'll need to indicate the electron orbitals. For this, we will first have to calculate the number of protons and neutrons present in this atom. Web lewis. Later on in this chapter and throughout this book we will see examples of organic ions called ‘carbocations’ and carbanions’, in which a carbon atom bears a positive or negative formal charge, respectively. It has symbol c and atomic number 6. Web steps of drawing the lewis structure of co 2 are explained in detail in this tutorial. Click to. The choice of modality varies depending on factors such as the information to be conveyed, grade level, student knowledge and experience, and the image itself. Web we will use this information to draw the bohr model of the carbon atom. Click to place an atom. Later on in this chapter and throughout this book we will see examples of organic. Web the small grey dot that follows your cursor is an atom (carbon). Click to place an atom. Web we will use this information to draw the bohr model of the carbon atom. In the first step, we will draw the nucleus of the carbon atom. In the lewis structure of co 2, you can see there are two double. In the first step, we will draw the nucleus of the carbon atom. During this course, you will view molecules written in all three forms. Web the atomic number for carbon is 6, so you'll need 6 protons, and in turn 6 electrons. You can simplify the formula by writing, for example, ch 3 or ch 2 instead of showing. Web this image can be made accessible with an image description, by creating a tactile graphic, or by printing a 3d object of the image. And each oxygen is six, and we have one of them, so six. Create a chain of carbon atoms. Each hydrogen is one valence electron, but we have two of them, so 1 times 2.. Web the bohr model of carbon is drawn with only two electron shells, the first shell contains 2 electrons and the second shell contains 4 electrons. Let’s draw and understand this lewis dot structure step by step. Carbon is neutral and its atomic number is 6, hence, the number of protons and electrons available for its bohr diagram is also 6. And each oxygen is six, and we have one of them, so six. Draw lewis structures of covalent molecules. Web lewis dot diagram of carbon. The carbon atom does not have a lone pair while both the oxygen atoms have 2 lone pairs. This video shows how to use the periodic table to draw lewis structures and figure out how many valence electrons an atom has. In this toolbar you can select from a number of elements, you can also pick an element from the periodic table using the last button. The choice of modality varies depending on factors such as the information to be conveyed, grade level, student knowledge and experience, and the image itself. Web drawing every bond and every atom is tedious, however, so chemists have devised several shorthand ways for writing structures. Web the atomic number for carbon is 6, so you'll need 6 protons, and in turn 6 electrons. Web carbon (from latin carbo 'coal') is a chemical element; Web the carbon atom (c) is at the center and it is surrounded by 2 oxygen atoms (o). Web the commonest way to draw structural formulae. Web this image can be made accessible with an image description, by creating a tactile graphic, or by printing a 3d object of the image.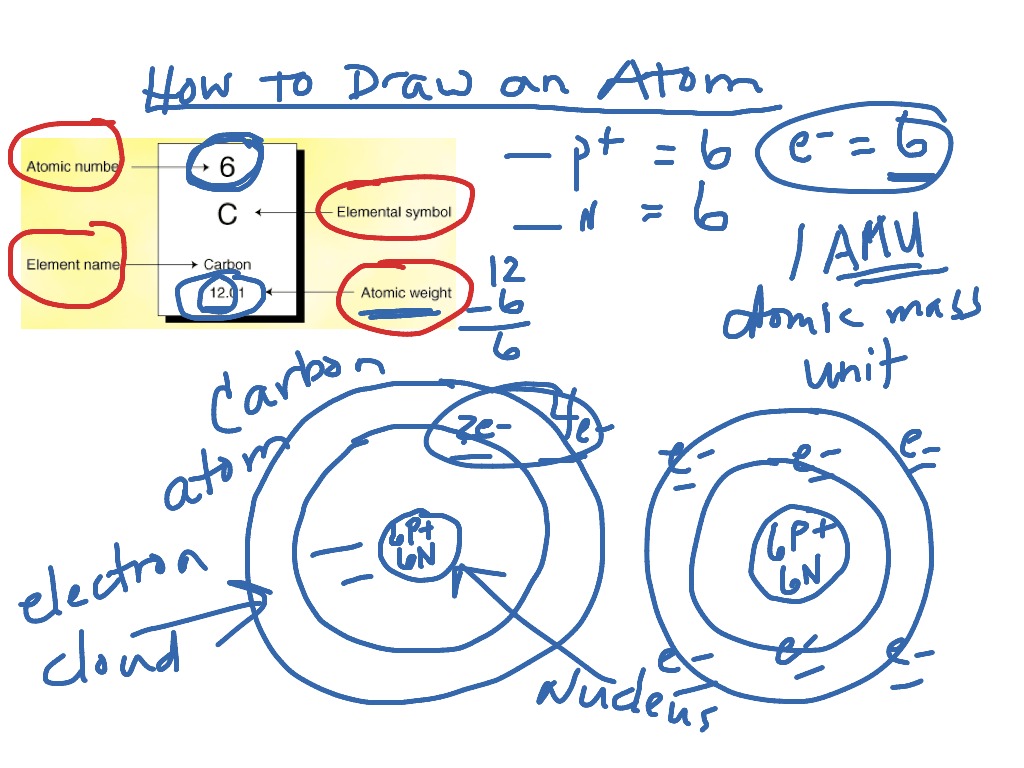
How to draw an atom of carbon Science ShowMe

Download Carbon, Atom, Atoms. RoyaltyFree Stock Illustration Image
REMC 22 / Figure App
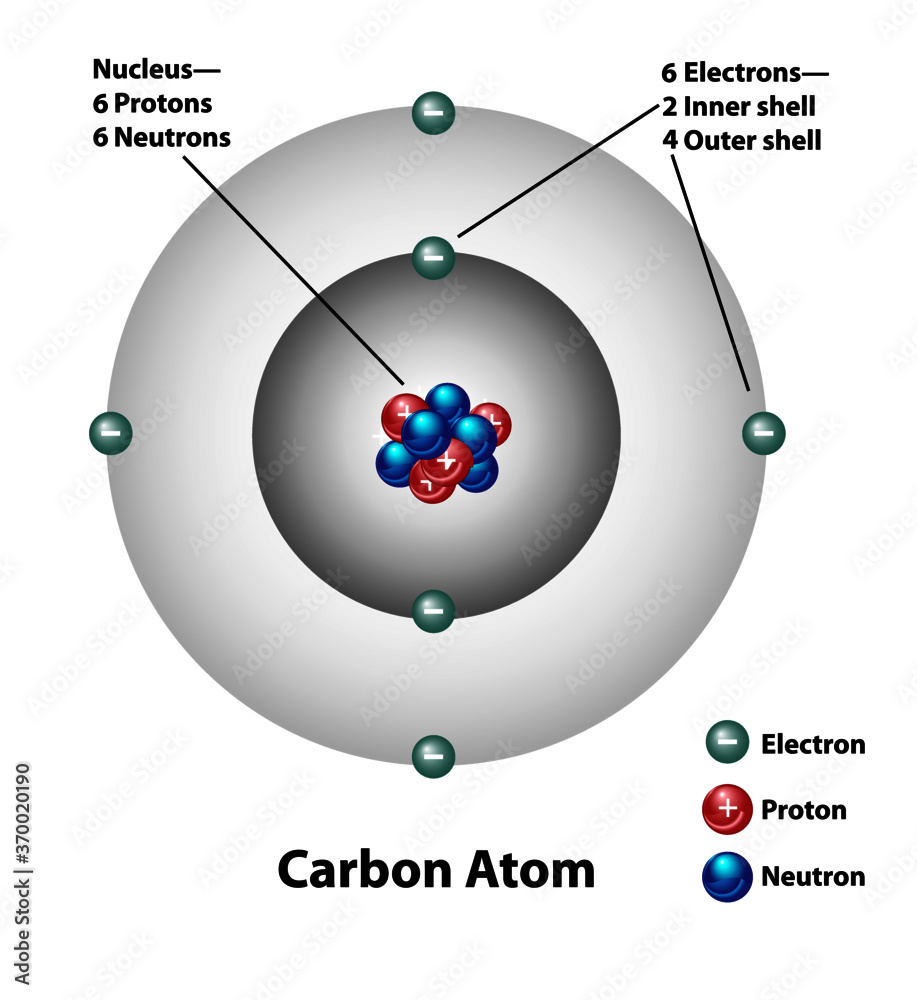
Molecular structure of a carbon atom. Electrons, protons, and neutrons

Labelled Diagram Of A Carbon Atom
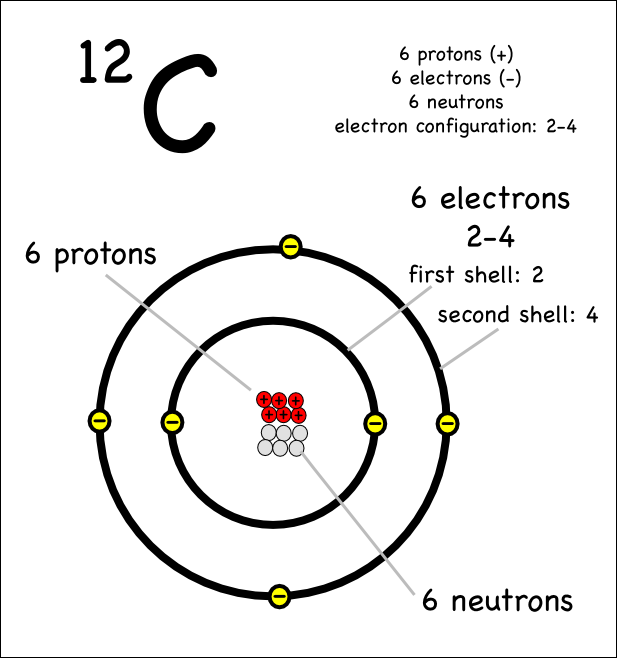
Drawing Atoms Montessori Muddle
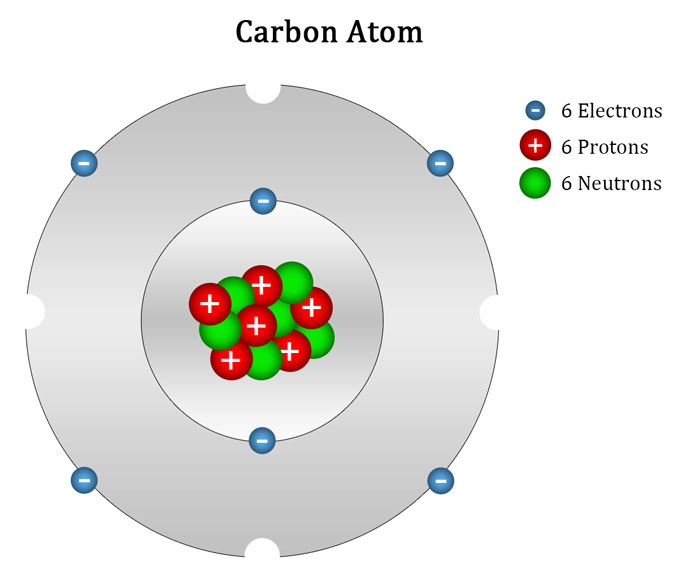
Carbon Atom Ascension Glossary

Premium Vector Carbon atom Bohr model

Carbon Atom Diagram
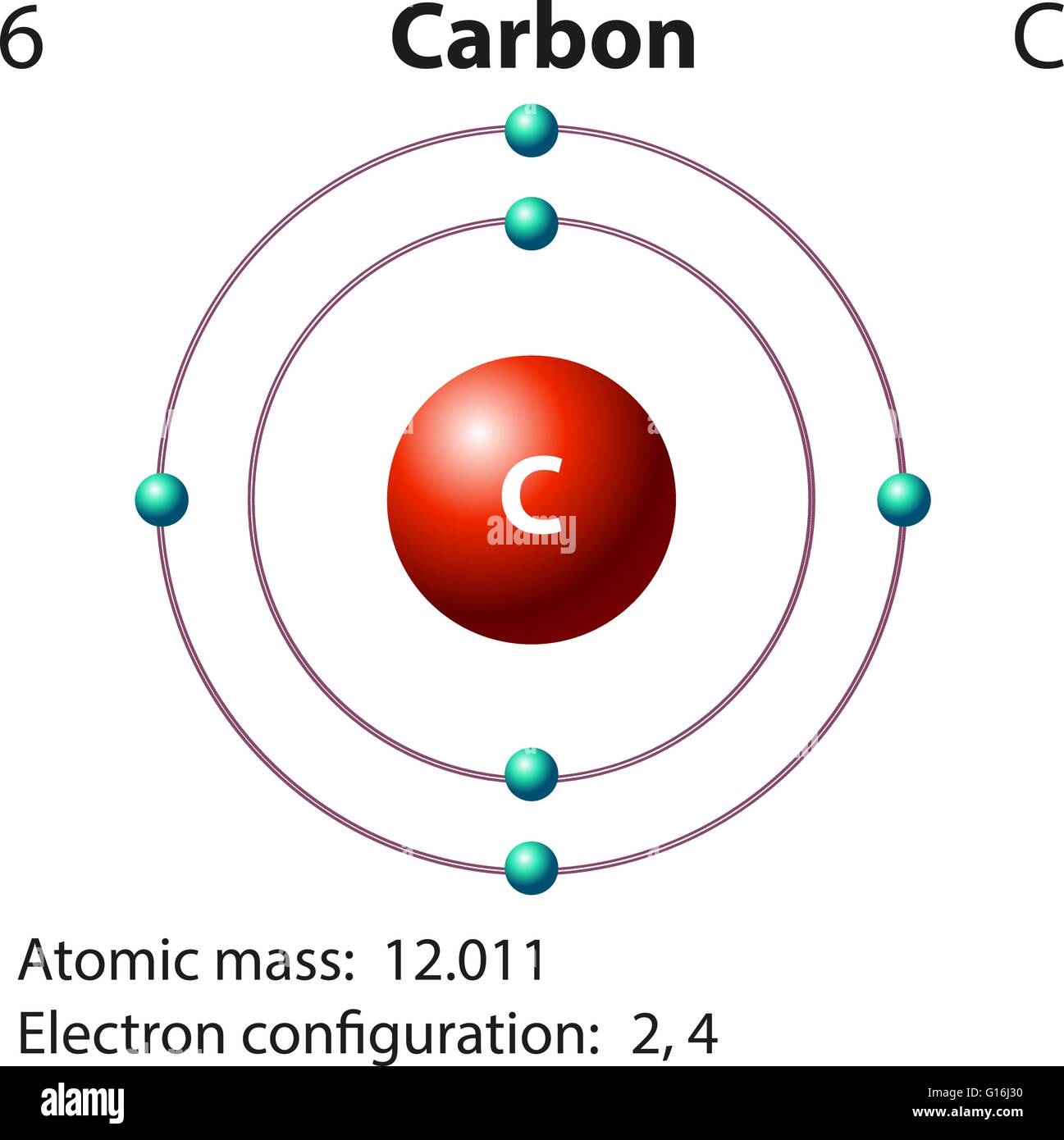
Carbon atom diagram hires stock photography and images Alamy
During This Course, You Will View Molecules Written In All Three Forms.
Web Explain How Electrons Are Shared Between Atoms.
As Long As It’s Carbon It Has Six Protons.
Web In Carbon Dioxide, The Carbon Atom Has Double Bonds To Oxygen On Both Sides (O=C=O).
Related Post: