Draw The Lewis Structure Of Pcl3
Draw The Lewis Structure Of Pcl3 - #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required. The following procedure can be used to draw lewis structure for simple molecules. = 4 + 1x4 = 8 valence electrons; Determine the total number of valence electrons in the molecule by adding the valence electrons of each atom. The phosphorus atom (p) is at the center and it is surrounded by 3 chlorine atoms (cl). Web i quickly take you through how to draw the lewis structure of pcl3, phosphorous trichloride. In this tutorial, we will learn how to draw the lewis structure of pcl 3 with all theories. The phosphorus atom has 1 lone pair and all the three chlorine atoms have 3 lone pairs. For \(\ce{pcl3}\), the electron dot diagram is as follows: Web lewis structure of pcl3 contains three single bonds between the phosphorus (p) atom and each chlorine (cl) atom. Web here are the steps to draw the pcl3 lewis structure: There are three single bonds between the phosphorus atom (p) and each of the chlorine atoms (cl). Web how to draw lewis structure. In pcl3, phosphorus has 5 valence electrons and each chlorine atom has 7 valence electrons, giving a total of 26 valence electrons. Pcl 3 has 5. The phosphorus atom (p) is at the center and it is surrounded by 3 chlorine atoms (cl). Web the first step is to draw the lewis structure of the molecule. Phosphorus trichloride is made up of one phosphorus atom and three chlorine atoms, having a chemical formula of pcl3. In this tutorial, we will learn how to draw the lewis. Let’s discuss each step in more detail. Web the lewis structure of pcl3 consists of a central phosphorus atom (p) and three external chlorine atoms (cl). This helps the atoms to gain stability. Web lewis structure of pcl3 contains three single bonds between the phosphorus (p) atom and each chlorine (cl) atom. This structure is also available as a 2d. Web use these steps to correctly draw the pcl 3 lewis structure: Phosphorus, on the periodic table, is in group 5, it has 5 valence electrons. In pcl3, phosphorus has 5 valence electrons and each chlorine atom has 7 valence electrons, giving a total of 26 valence electrons. Phosphorus trichloride is made up of one phosphorus atom and three chlorine. Ch 4 has 4 valence electrons in c, and 1 in each of the four h: Count the total number of valence electrons to find out […] kcl lewis structure. Chlorine, group 7, but we have three of those so we have 5 plus 7 (times 3 is 21) is 26 valence electrons. Web how to draw lewis structure. Web. Web let's do the lewis structure for pcl3. Chlorine, group 7, but we have three of those so we have 5 plus 7 (times 3 is 21) is 26 valence electrons. Web how to draw a lewis structure for pcl3? Drawing lewis structures for bf3, pf3 and brf3; = 4 + 1x4 = 8 valence electrons; = 5 + 7x3 = 26 valence electrons 74k views 4 years ago. B) draw the lewis structure. I also go over hybridization, shape and bond angle. Web let's do the lewis structure for pcl3. The lewis structure of any compound helps us to find out the number of bonds, types of bonds, and the interaction between the atoms in a compound. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. B) draw the lewis structure. The phosphorus atom has. Web pcl3 molecular electron geometry, lewis structure, bond angles and hybridization. Web the first step is to draw the lewis structure of the molecule. Web lewis structure of pcl3 contains three single bonds between the phosphorus (p) atom and each chlorine (cl) atom. = 4 + 1x4 = 8 valence electrons; Web explore the molecular structure and formula of phosphorus. Web a video explanation of how to draw the lewis dot structure for phosphorous trichloride, along with information about the compound including formal charges, polarity, hybrid orbitals, shape,. The lone electron pairs on the cl atoms are omitted for clarity. Web how to draw the lewis structure of pcl3 (phosphorus trichloride) chemistnate. Ch 4 has 4 valence electrons in c,. Web how to draw lewis structure. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required. Web i quickly take you through how to draw the lewis structure of pcl3, phosphorous trichloride. In this tutorial, we will learn how to draw the lewis structure of pcl 3 with all theories. Web let's do the lewis structure for pcl3. Web the pcl3 lewis structure showcases the interaction between phosphorus and chlorine atoms, revealing how they share electrons to achieve stability. Web here are the steps to draw the pcl3 lewis structure: Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Web draw the lewis structures of ch 4, pcl 3, co 2, and hcn. A lewis structure mainly focuses on fulfilling the octet of the atoms in a compound. Phosphorus trichloride is made up of one phosphorus atom and three chlorine atoms, having a chemical formula of pcl3. Web the first step is to draw the lewis structure of the molecule. Web drawing lewis structures for molecules with one central atom: Pcl 3 has 5 valence electros in p and 7 in each of the three cl: The following procedure can be used to draw lewis structure for simple molecules. This helps the atoms to gain stability.
What Is Pcl3 Lewis Structure?

Explain How To Draw The Lewis Structure For Pcl3
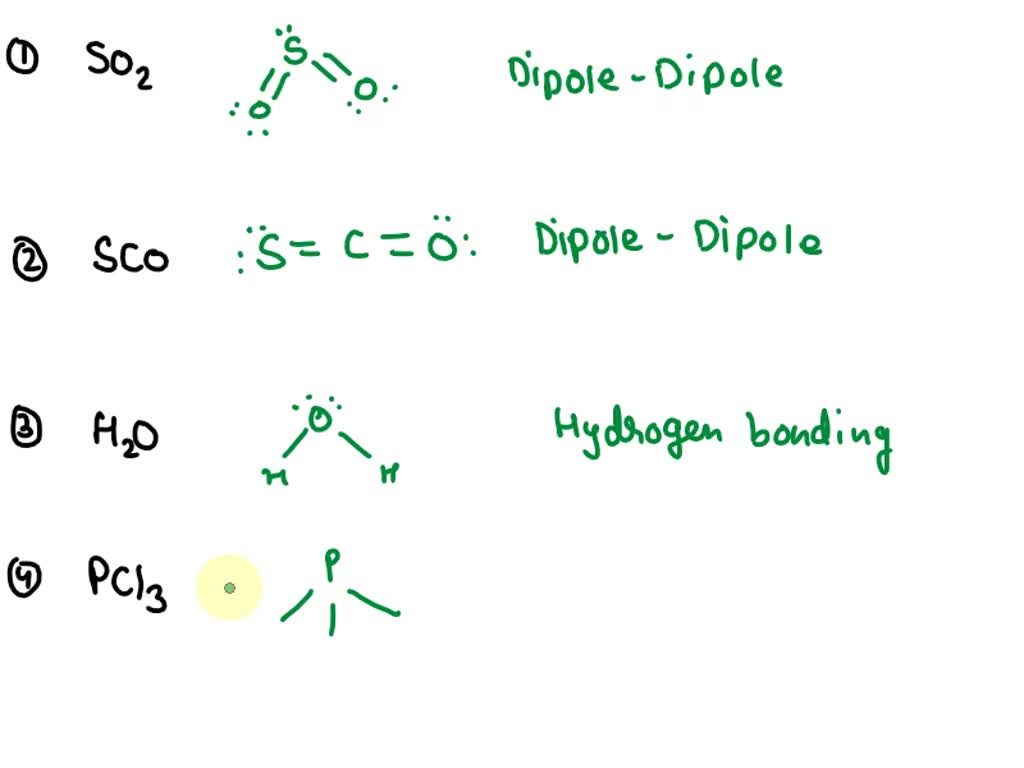
Explain How To Draw The Lewis Structure For Pcl3

Explain How To Draw The Lewis Structure For Pcl3
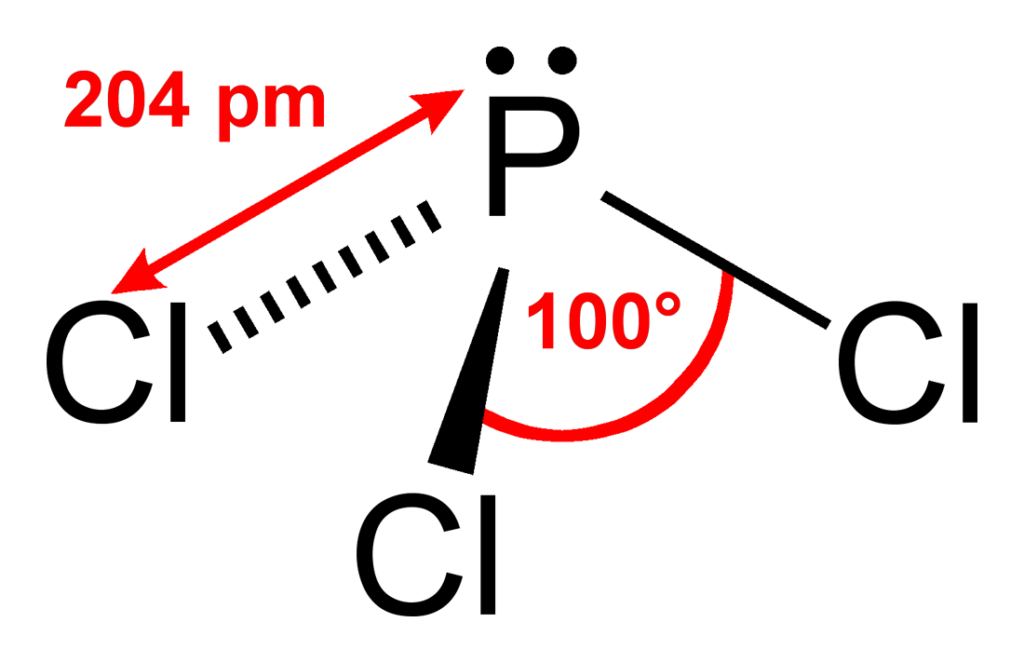
PCl3 Molecular Electron Geometry, Lewis Structure, Bond Angles and

How to draw PCl3 Lewis Structure? Science Education and Tutorials

PCl3 Lewis Structure How to Draw the Lewis Structure for PCl3
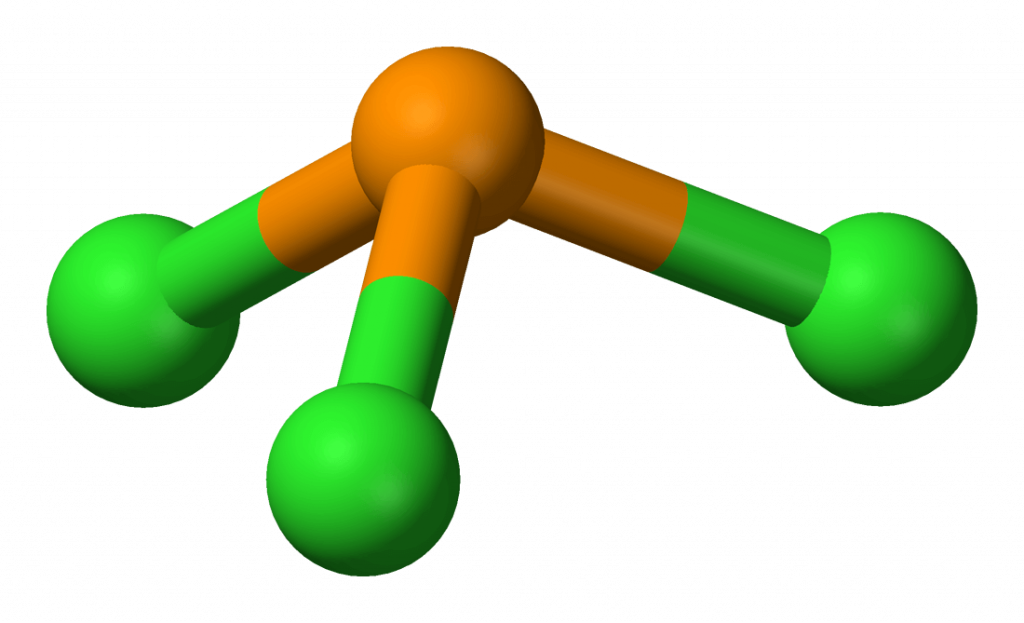
PCL3 Molecular Electron Geometry, Lewis Structure, Bond Angles and

Pcl3 Electron Geometry And Molecular Geometry Drawing Easy

PCl3 Lewis StructureLewis Structure of PCl3 (Phosphorus Trichloride
Web A Video Explanation Of How To Draw The Lewis Dot Structure For Phosphorous Trichloride, Along With Information About The Compound Including Formal Charges, Polarity, Hybrid Orbitals, Shape,.
The Phosphorus Atom (P) Is At The Center And It Is Surrounded By 3 Chlorine Atoms (Cl).
Web Use These Steps To Correctly Draw The Pcl 3 Lewis Structure:
Phosphorus, On The Periodic Table, Is In Group 5, It Has 5 Valence Electrons.
Related Post: