Draw The Lewis Structure Of Clbr3 Showing All Lone Pairs
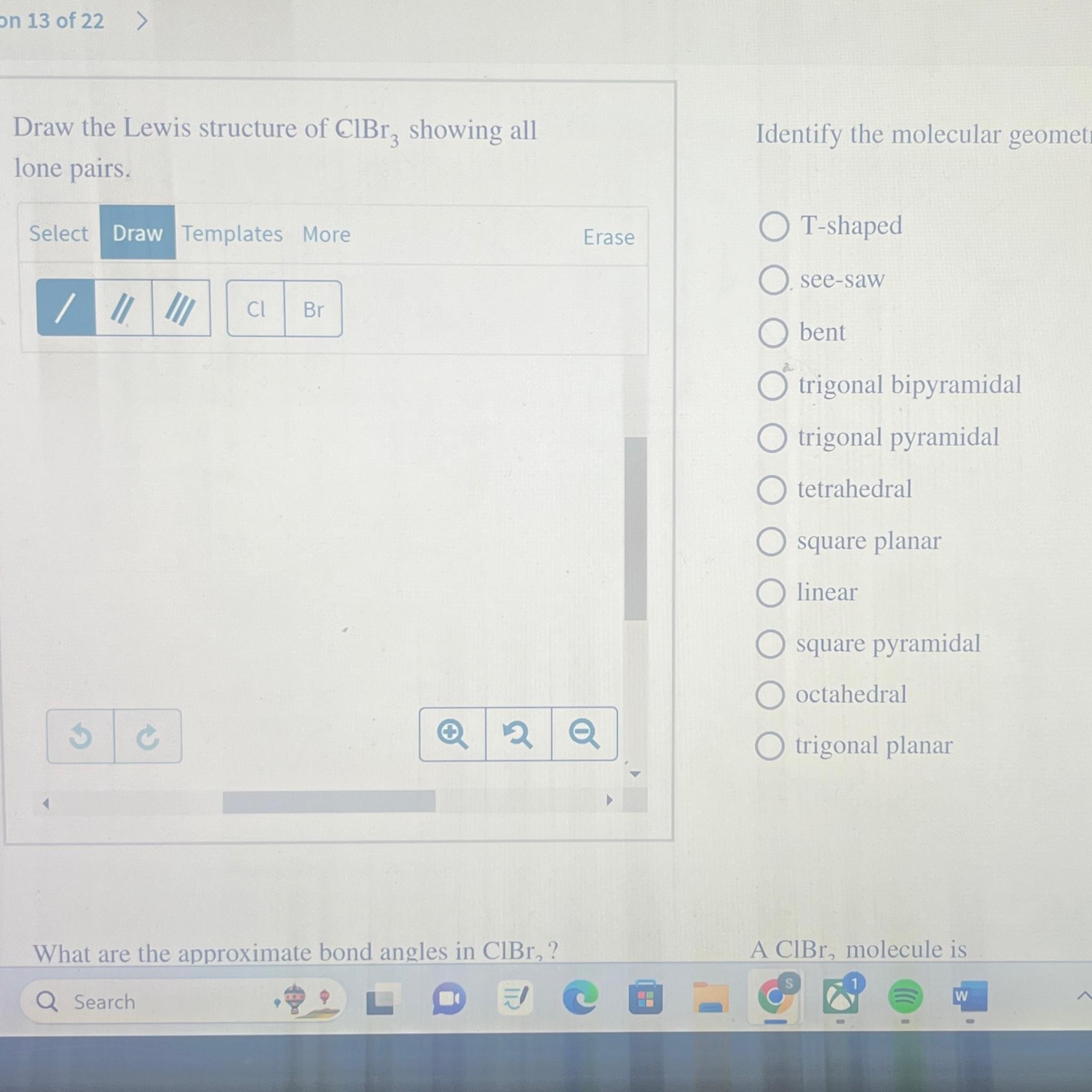
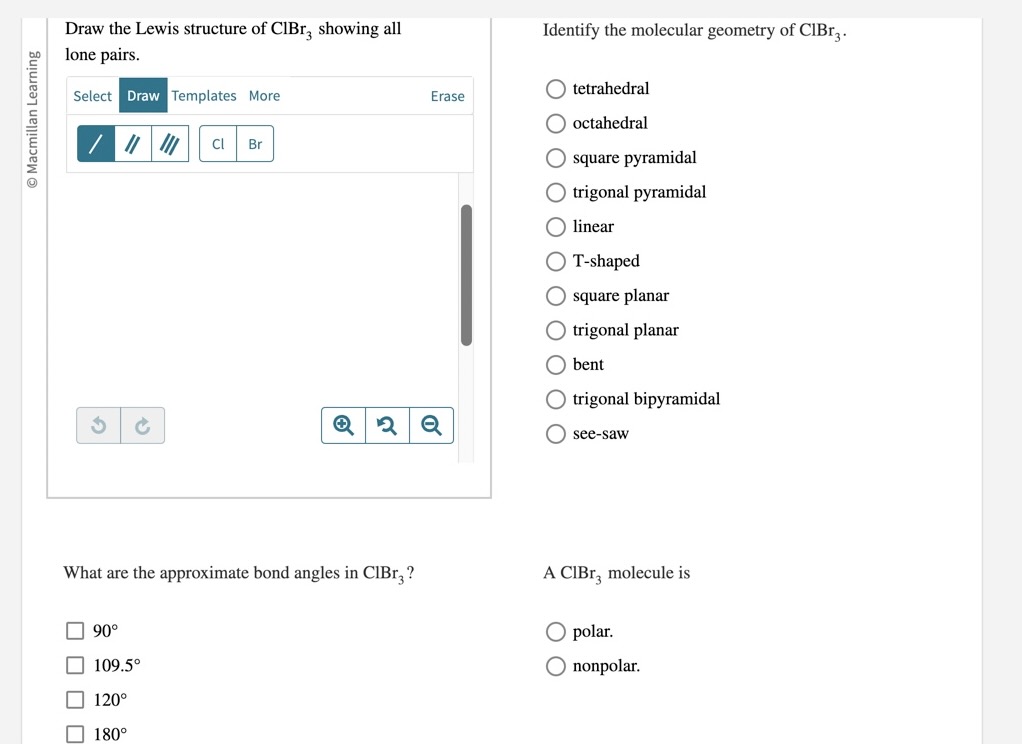
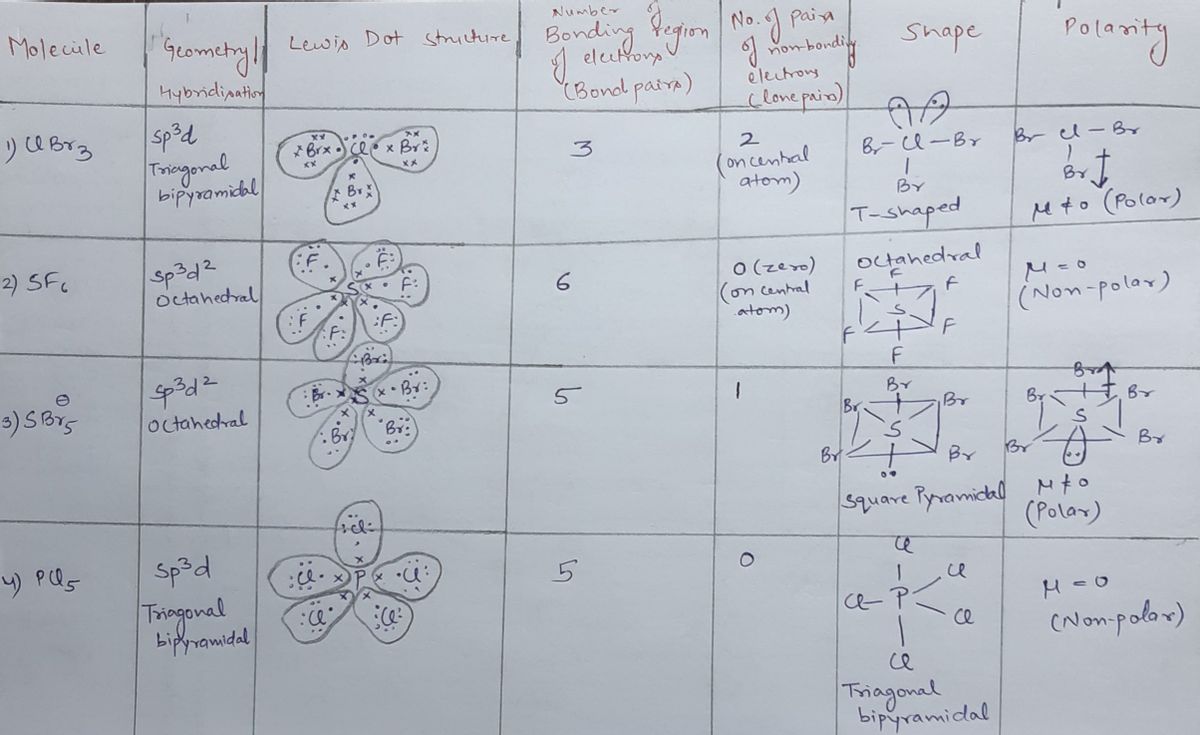
Draw The Lewis Structure Of Clbr3 Showing All Lone Pairs - What are the approximate bond angles in c l b r 2 ? There are 2 steps to solve this one. Draw the lewis structure of. Web the lewis structure of clbr3 includes central atom bromine (br) sharing one electron pair with each of three chlorine atoms (cl). This is how we predict and draw the lewis. Web draw the lewis structure of clbr: Web answer to draw the lewis structure of clbr3 showing all lone pairs. Web your solution’s ready to go! Web draw the lewis structure of clbr3 showing all lone pairs. Web as it is visible, 2 lone pairs are surrounding the bromine atom whereas there are 3lone pairs on each chlorine atom. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. This is how we predict and draw the lewis. There are 2 steps to solve this one. Web start filling in the gaps now. What are the approximate bond angles in c l b r 2 ? Draw the lewis structure of. So, let’s calculate this first. Draw the lewis structure of clbr3 showing all lone pairs. Draw the lewis structure of clbr3 showing all lone. Web as it is visible, 2 lone pairs are surrounding the bromine atom whereas there are 3lone pairs on each chlorine atom. Web so, three lone lone pairs (or 6 electrons) are left on each bromine atom. What are the approximate bond angles in clbr3? There are 2 steps to solve this one. Web as it is visible, 2 lone pairs are surrounding the bromine atom whereas there are 3lone pairs on each chlorine atom. In this way, all atoms in clbr3. Web a lewis structure is a representation of covalent molecules (or polyatomic ions) where all the valence electrons are shown distributed about the bonded atoms as either shared. In this way, all atoms in clbr3 molecule gains octet of electrons and becomes stable. This is how we predict and draw the lewis. Identify the molecular geometry of clbr3. In order. Web start filling in the gaps now. What are the approximate bond angles in clbr3? (valence electrons are the number of electrons present in the outermost shell of an atom). Draw the lewis structure of clbr3 showing all lone. Web this widget gets the lewis structure of chemical compounds. Each cl atom interacts with eight valence. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Your solution’s ready to go! In this way, all atoms in clbr3 molecule gains octet of electrons and becomes stable. Web a lewis structure is a representation of covalent molecules (or polyatomic ions) where all the valence electrons. Draw the lewis structure of clbr3 showing all lone pairs. There are 2 steps to solve this one. In this way, all atoms in clbr3 molecule gains octet of electrons and becomes stable. What are the approximate bond angles in clbr3? Draw the lewis structure of clbr3 showing all lone. Web a lewis structure is a representation of covalent molecules (or polyatomic ions) where all the valence electrons are shown distributed about the bonded atoms as either shared. There are 2 steps to solve this one. Web as it is visible, 2 lone pairs are surrounding the bromine atom whereas there are 3lone pairs on each chlorine atom. Draw the. Each cl atom interacts with eight valence. Web so, three lone lone pairs (or 6 electrons) are left on each bromine atom. Identify the molecular geometry of clbr3. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web answer to draw the lewis structure of clbr3 showing all lone pairs. Web draw the lewis structure of clbr: Here, the given molecule is clbr3. Web a lewis structure is a representation of covalent molecules (or polyatomic ions) where all the valence electrons are shown distributed about the bonded atoms as either shared. What are the approximate bond angles in c l b r 2 ? Web the lewis structure of clbr3. Each cl atom interacts with eight valence. Draw the lewis structure of clbr3 showing all lone pairs. In this question we have to draw the lewis structure of clbr3 and we have to tell the molecular geometry and approximate bond angle. Web so, three lone lone pairs (or 6 electrons) are left on each bromine atom. Web the lewis structure of clbr3 includes central atom bromine (br) sharing one electron pair with each of three chlorine atoms (cl). Web start filling in the gaps now. Here, the given molecule is clbr3. (valence electrons are the number of electrons present in the outermost shell of an atom). Web a lewis structure is a representation of covalent molecules (or polyatomic ions) where all the valence electrons are shown distributed about the bonded atoms as either shared. Web your solution’s ready to go! So, let’s calculate this first. This is how we predict and draw the lewis. Web as it is visible, 2 lone pairs are surrounding the bromine atom whereas there are 3lone pairs on each chlorine atom. In this way, all atoms in clbr3 molecule gains octet of electrons and becomes stable. What are the approximate bond angles in c l b r 2 ? Web this widget gets the lewis structure of chemical compounds.[Solved] Draw the Lewis structure of ClBr3 with lone pairs. Course Hero
Solved on 13 of 22>Draw the Lewis structure of ClBr3
Draw The Lewis Structure Of Clbr3 Showing All Lone Pairs Pratt Blog
Solved Draw the Lewis structure of ClBr3 showing all Inna
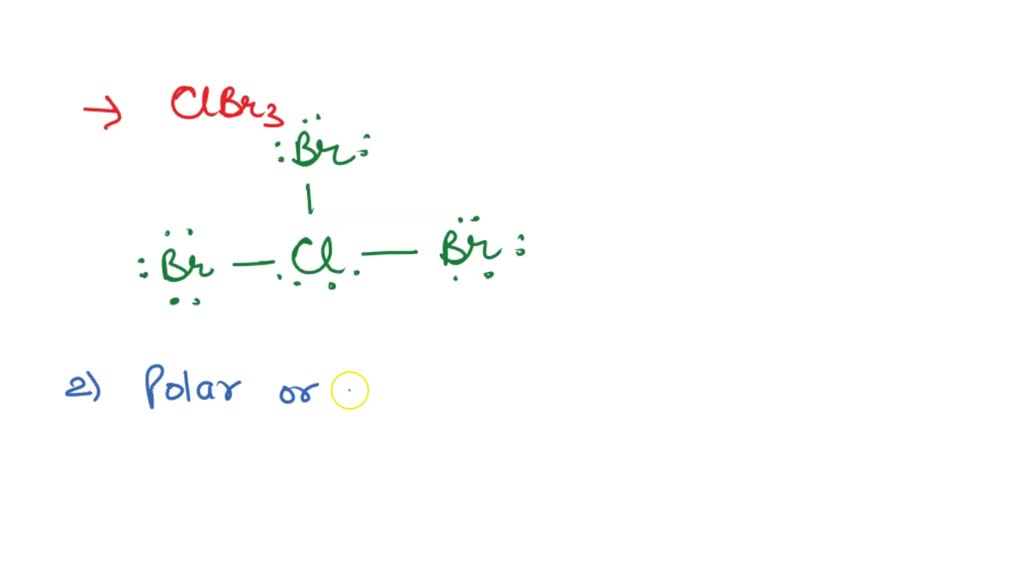
SOLVED Draw the Lewis structure for PF, . including lone pairs. Select

Answered Draw the Lewis structure of CIBr3… bartleby

Draw The Lewis Structure Of Clbr3 Showing All Lone Pairs 55+ Pages
Draw The Lewis Structure Of Clbr3 Showing All Lone Pairs Drawing

SOLVED 'Draw the lewis structure of ClBr3 showing all lone pairs.

OneClass Draw the lewis structure of ClBr3 showing all lone pairs.
Get The Free Lewis Structure Finder Widget For Your Website, Blog, Wordpress, Blogger, Or Igoogle.
Identify The Molecular Geometry Of Clbr3.
In Order To Draw The Lewis Structure Of Clbr3, First Of All You Have To Find The Total Number Of Valence Electrons Present In The Clbr3 Molecule.
Web Answer To Draw The Lewis Structure Of Clbr3 Showing All Lone Pairs.
Related Post: