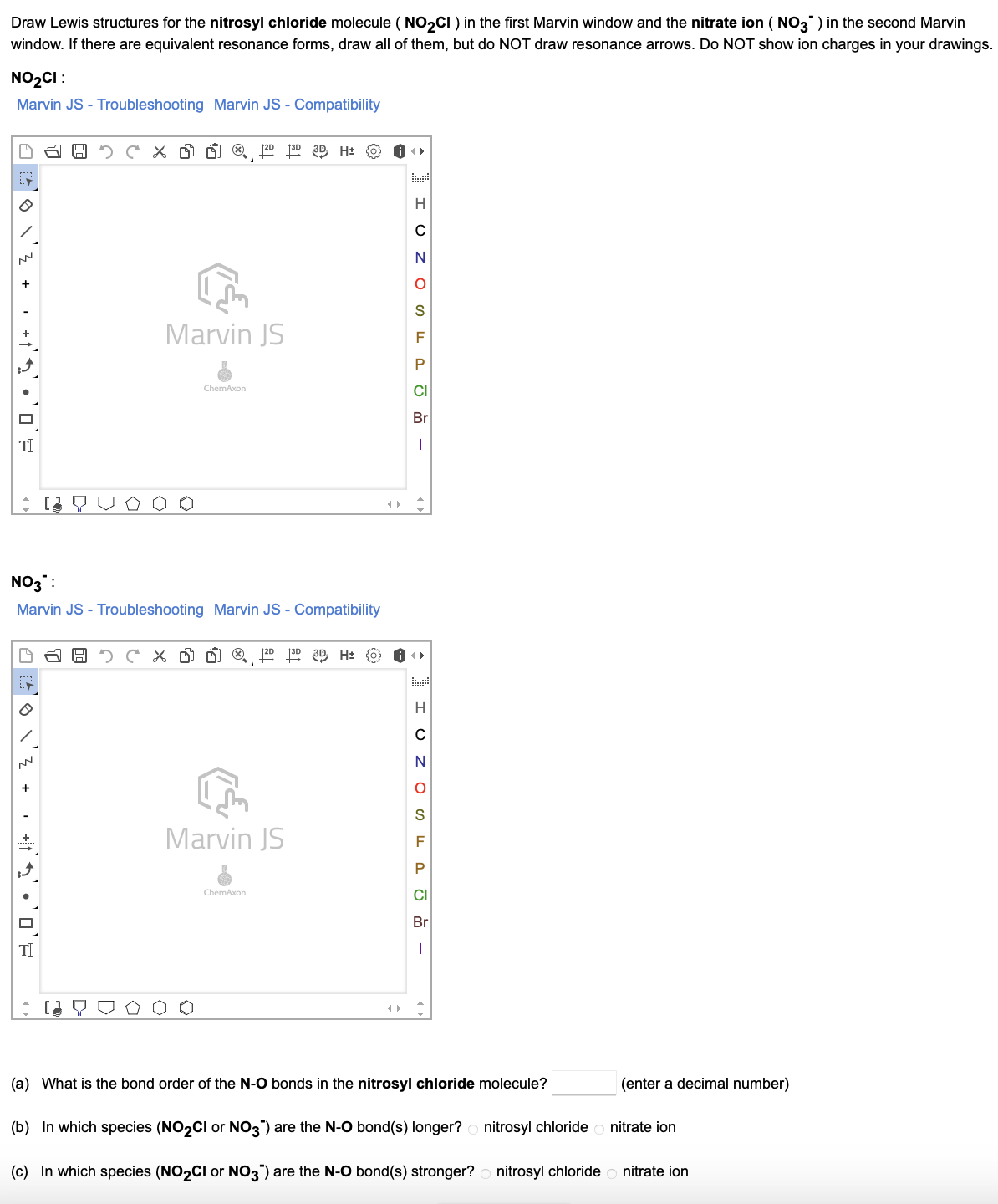
Draw The Lewis Structure For The Nitrosyl Chloride Molecule
Draw The Lewis Structure For The Nitrosyl Chloride Molecule - In the first structure, nitrogen is bonded to chlorine with a single bond, while in the second. Web because nitrogen is less electronegative than oxygen or chlorine, it is the central atom. Draw the lewis structure for the nitrosyl chloride (noci) molecule. Web nocl (nitrosyl chloride) has one nitrogen atom, one oxygen atom, and one chlorine atom. The n atom (group 15) has 5 valence electrons, the o atom (group 16) has 6 valence. Web nitrosyl chloride (nocl) can have two possible lewis structures. For the no2cl structure use the periodic table to find the total number of valence electrons for. Web drawing lewis structures for molecules with one central atom: Your solution’s ready to go! Web nitrogen (n), which is linked to both oxygen (o) and chlorine (cl), is the core atom in the lewis structure of nitrosyl chloride (nocl). Web nitrosyl chloride is the chemical compound with the formula nocl. In the nocl lewis structure, there is a double bond between nitrogen. Here’s the best way to solve it. Web because nitrogen is less electronegative than oxygen or chlorine, it is the central atom. Web nocl (nitrosyl chloride) has one nitrogen atom, one oxygen atom, and one chlorine atom. Web nitrogen (n), which is linked to both oxygen (o) and chlorine (cl), is the core atom in the lewis structure of nitrosyl chloride (nocl). The n atom (group 15) has 5 valence electrons, the o atom (group 16) has 6 valence. Web all atoms now have octet configurations. Web nitrosyl chloride (nocl) can have two possible lewis structures. Web. Web draw the lewis structure of nocl, nitrosyl chloride, and then determine the number of nonbonding electron pairs on the central atom. Draw lewis structure (s) showing all possible equivalent. In the first structure, nitrogen is bonded to chlorine with a single bond, while in the second. Web all atoms now have octet configurations. For the no2cl structure use the. With oxygen, nitrogen forms a. Web all atoms now have octet configurations. Web nitrosyl chloride is the chemical compound with the formula nocl. Draw the lewis structure for the nitrosyl chloride (noci) molecule. In the nocl lewis structure, there is a double bond between nitrogen. Web draw the lewis structure of nocl, nitrosyl chloride, and then determine the number of nonbonding electron pairs on the central atom. Draw lewis structure (s) showing all possible equivalent. The n atom (group 15) has 5 valence electrons, the o atom (group 16) has 6 valence. Web all atoms now have octet configurations. Web nitrosyl chloride (nocl) can have. Web all atoms now have octet configurations. Web nitrosyl chloride is the chemical compound with the formula nocl. There are 3 steps to solve this one. Web nitrosyl chloride (nocl) can have two possible lewis structures. Web all atoms now have octet configurations. Web all atoms now have octet configurations. The n atom (group 15) has 5 valence electrons, the o atom (group 16) has 6 valence. In the nocl lewis structure, there is a double bond between nitrogen. Your solution’s ready to go! Web draw the lewis structure for the nitrosyl chloride (nocl) molecule. There are 3 steps to solve this one. Web all atoms now have octet configurations. Web all atoms now have octet configurations. In the first structure, nitrogen is bonded to chlorine with a single bond, while in the second. Your solution’s ready to go! In the first structure, nitrogen is bonded to chlorine with a single bond, while in the second. It is a yellow gas that is commonly encountered as a component of aqua regia, a mixture of 3 parts. Web nitrogen (n), which is linked to both oxygen (o) and chlorine (cl), is the core atom in the lewis structure of nitrosyl. Web all atoms now have octet configurations. It is a yellow gas that is commonly encountered as a component of aqua regia, a mixture of 3 parts. Web nitrosyl chloride (nocl) can have two possible lewis structures. With oxygen, nitrogen forms a. Web because nitrogen is less electronegative than oxygen or chlorine, it is the central atom. Draw the lewis structure for the nitrosyl chloride (noci) molecule. Web drawing lewis structures for molecules with one central atom: Web because nitrogen is less electronegative than oxygen or chlorine, it is the central atom. Web nitrosyl chloride (nocl) can have two possible lewis structures. Web all atoms now have octet configurations. In the first structure, nitrogen is bonded to chlorine with a single bond, while in the second. Web nocl (nitrosyl chloride) has one nitrogen atom, one oxygen atom, and one chlorine atom. In the nocl lewis structure, there is a double bond between nitrogen. There are 3 steps to solve this one. Your solution’s ready to go! Web nitrogen (n), which is linked to both oxygen (o) and chlorine (cl), is the core atom in the lewis structure of nitrosyl chloride (nocl). Web all atoms now have octet configurations. Web nitrosyl chloride is the chemical compound with the formula nocl. Web draw the lewis structure for the nitrosyl chloride (nocl) molecule. Draw lewis structure (s) showing all possible equivalent. Here’s the best way to solve it.
What Is The Lewis Structure Of Nitrosyl Chloride Nocl Socratic My XXX
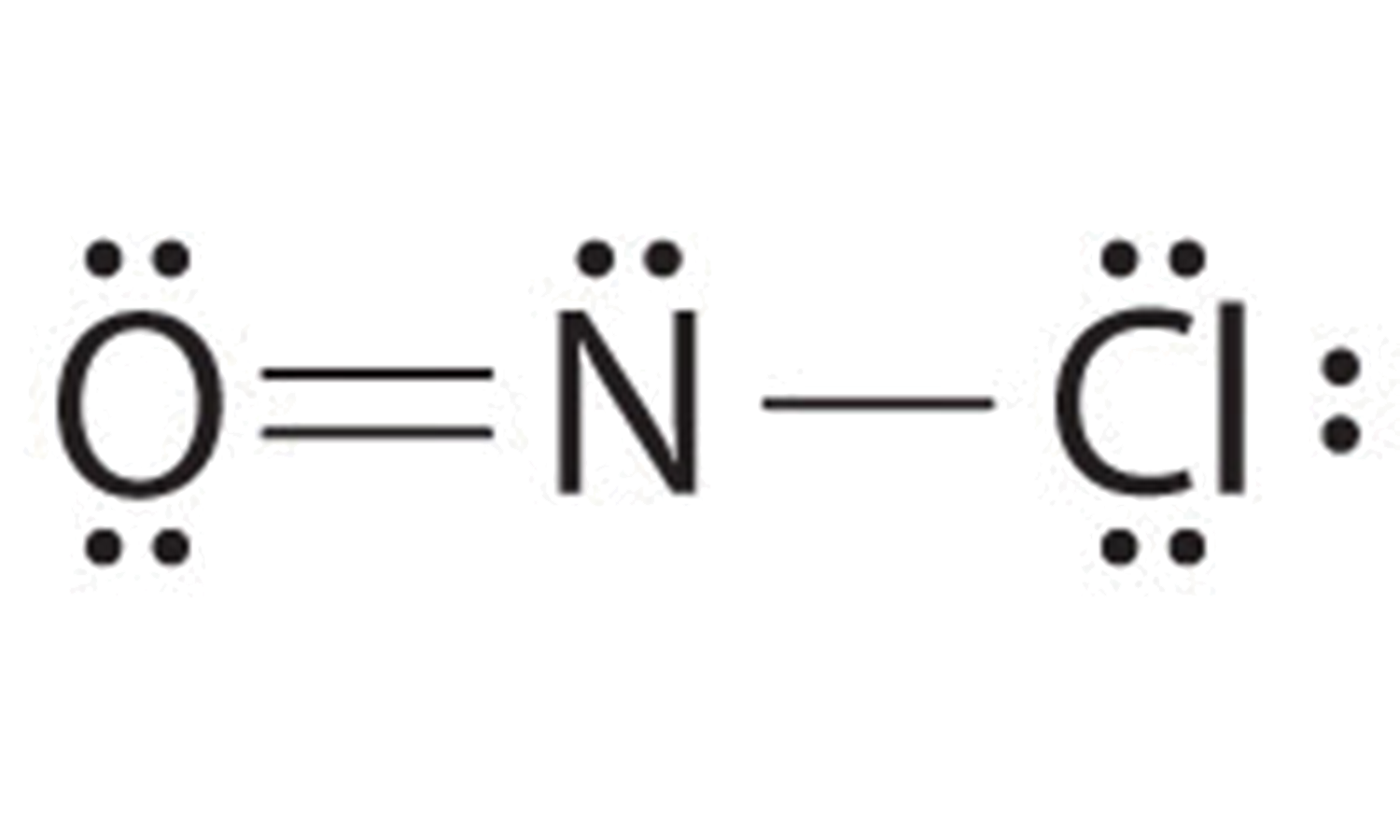
9.7 Lewis Structures Chemistry LibreTexts
Solved Draw Lewis structures for the nitrosyl chloride

What is the Lewis structure of nitrosyl chloride, NOCl? CBSE Tuts
[Solved] Consider the Lewis structure of nitrosyl chloride (NOCl) From
Solved Draw the Lewis structure for the nitrosyl chloride

Nitrosyl chloride 2696926

Oncl Lewis Structure
[Solved] Draw the Lewis structure for the nitrosyl chloride (NOC
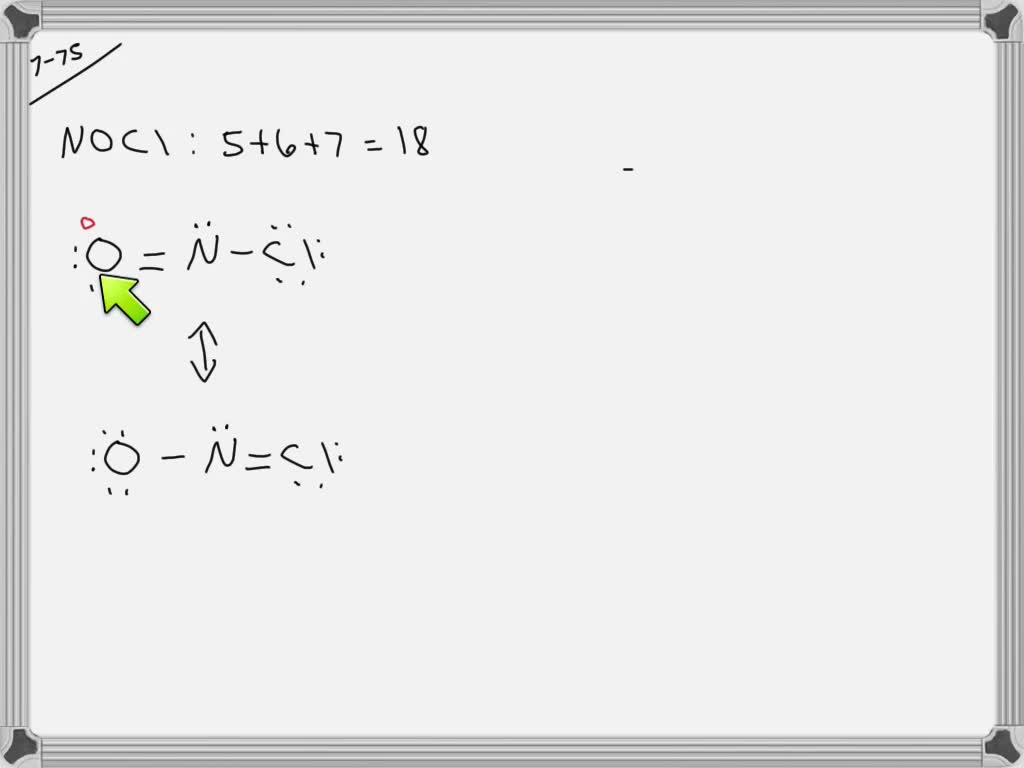
SOLVED Draw two electrondot resonance structures that obey the octet
With Oxygen, Nitrogen Forms A.
The N Atom (Group 15) Has 5 Valence Electrons, The O Atom (Group 16) Has 6 Valence.
For The No2Cl Structure Use The Periodic Table To Find The Total Number Of Valence Electrons For.
Web Draw The Lewis Structure Of Nocl, Nitrosyl Chloride, And Then Determine The Number Of Nonbonding Electron Pairs On The Central Atom.
Related Post: