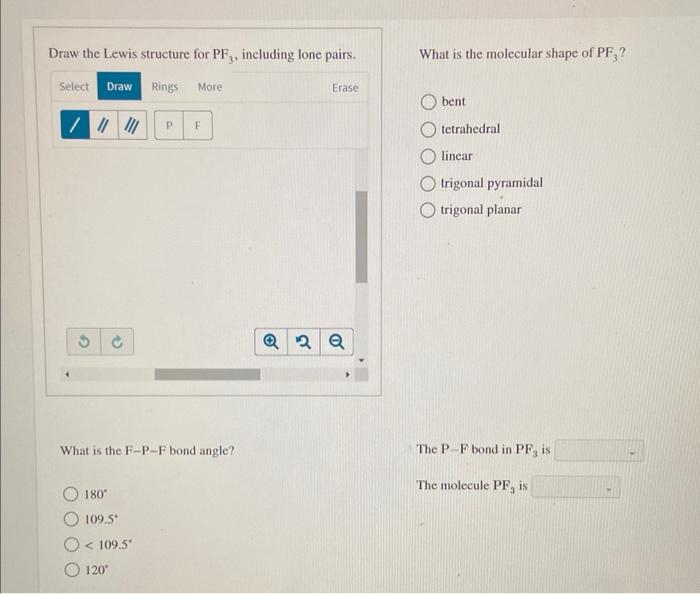
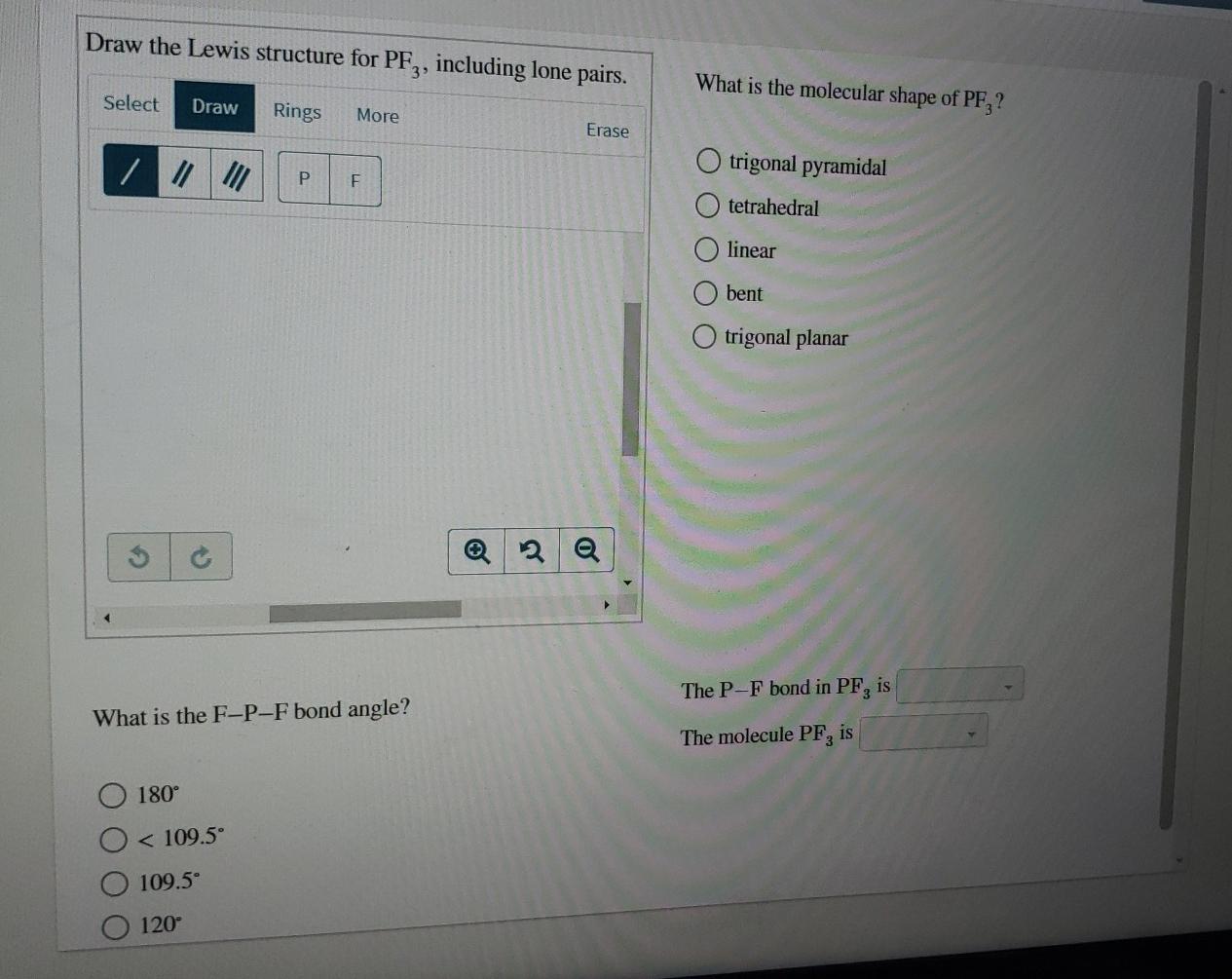
Draw The Lewis Structure For Pf3 Including Lone Pairs
Draw The Lewis Structure For Pf3 Including Lone Pairs - Web draw the lewis structure for pf 3 , including lone pairs. Web in the pf 3 lewis structure phosphorus (p) is the least electronegative so it goes in the center. In the pf 3 molecule, there will be a lone pair over phosphorus present in sp 3 hybridized orbital and three fluorine have. 7 valence electrons each (3 f atoms, total of 21 valence electrons). The phosphorus atom has one lone pair, represented by two dots, on its. Web we show you how to draw the lewis structure and determine the molecular geometry for phosphorus trifluoride (pf3). Trigonal planar tetrahedral bent linear trigonal pyramidal what is the f − p − f. Web the covalent bonds are drawn as short lines in this book, and one covalent bond means one pair of bonding electrons, that is 2 electrons. The phosphorus atom (p) is at the center and it is surrounded. In the lewis structure for pf 3 there are a total of 26 valence electrons. Web lewis structure of a compound shows, bonding between two atoms in a molecule, along with their lone pairs, shown as dots. Web lewis structure of pf3 contains three single bonds between the phosphorus (p) atom and each fluorine (f) atom. Draw the lewis dot structure for pf3. In the pf 3 molecule, there will be a lone pair over. Web the lewis structure of pf3 can be represented as follows:. Web the p−f bond in pf3 is 180∘ the molecule pf3 is 109.5∘ <109.5∘ 120∘ this problem has been solved! Web drawing lewis structures for molecules with one central atom: Web your solution’s ready to go! Web in the lewis structure of pf3, the central phosphorus atom has one. Draw the molecule by placing atoms on the grid and. Web the p−f bond in pf3 is 180∘ the molecule pf3 is 109.5∘ <109.5∘ 120∘ this problem has been solved! Phosphorus forms single bonds with each of the three fluorine. The phosphorus atom has one lone pair, represented by two dots, on its. What is the molecular shape of pf. Web your solution’s ready to go! Web lewis structure of pf3 contains three single bonds between the phosphorus (p) atom and each fluorine (f) atom. Web the covalent bonds are drawn as short lines in this book, and one covalent bond means one pair of bonding electrons, that is 2 electrons. Web drawing lewis structures for molecules with one central. Web lewis structure of pf3 contains three single bonds between the phosphorus (p) atom and each fluorine (f) atom. You'll get a detailed solution from a subject matter expert that helps you. Web draw the lewis structure for pf 3 , including lone pairs. Web alright, so we have our lewis structure and we determined the hybridization is sp three. For the pf3 structure use the periodic table to find the total number of valence. The phosphorus atom (p) is at the center and it is surrounded. Draw the lewis dot structure for pf3. Trigonal planar tetrahedral bent linear trigonal pyramidal what is the f − p − f. Web in the pf 3 lewis structure phosphorus (p) is the. For the pf3 structure use the periodic table to find the total number of valence. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the. Draw the lewis dot structure for pf3. Web the p−f bond in pf3 is 180∘ the molecule pf3 is 109.5∘ <109.5∘ 120∘ this. Web to properly draw the pf 3 lewis structure, follow these steps: Web drawing lewis structures for molecules with one central atom: Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web draw the lewis structure for pf 3 , including lone pairs. Web we show you how to draw the lewis. In the pf 3 molecule, there will be a lone pair over phosphorus present in sp 3 hybridized orbital and three fluorine have. You'll get a detailed solution from a subject matter expert that helps you. Phosphorus forms single bonds with each of the three fluorine. Web the p−f bond in pf3 is 109.5∗ the molecule pf3 is 120∗ <109.5∘. Draw a lewis structure for. Web lewis structure of pf3 contains three single bonds between the phosphorus (p) atom and each fluorine (f) atom. Web drawing lewis structures for molecules with one central atom: Web the lewis structure for pf3 shows a single phosphorus atom with three fluorine atoms bonded to it. In the pf 3 molecule, there will be. Web hello everyone!we are back with yet another video that can help you determine the lewis structure of pf3 molecule. Web lewis structure of a compound shows, bonding between two atoms in a molecule, along with their lone pairs, shown as dots. In the lewis structure for pf 3 there are a total of 26 valence electrons. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the. You'll get a detailed solution from a subject matter expert that helps you. Web the lewis structure of pf3 can be represented as follows:. The following image shows the lewis structure of. Web to properly draw the pf 3 lewis structure, follow these steps: Trigonal planar tetrahedral bent linear trigonal pyramidal what is the f − p − f. 7 valence electrons each (3 f atoms, total of 21 valence electrons). Web your solution’s ready to go! Web drawing lewis structures for molecules with one central atom: So this appears to be the first. Web in the lewis structure of pf3, the central phosphorus atom has one lone pair of electrons represented by two dots. Draw a lewis structure for. Write a lewis structure for the phosphorus trifluoride molecule, pf3.[Solved] Draw the Lewis structure for PF3 on your shown work (P is the

Draw The Lewis Structure For Pf3 Including Lone Pairs Drawing.rjuuc

How to Draw the Lewis Dot Structure for PF3 Phosphorus trifluoride
SOLVED Draw the Lewis structure for PF3, including lone pairs.

Draw The Lewis Structure For Pf3 Including Lone Pairs Drawing.rjuuc
Solved Draw the Lewis structure for PF3, including lone

SOLVED Draw the Lewis structure for PF3, including lone pairs.
draw the lewis structure for pf3 including lone pairs bettinaniedermaier
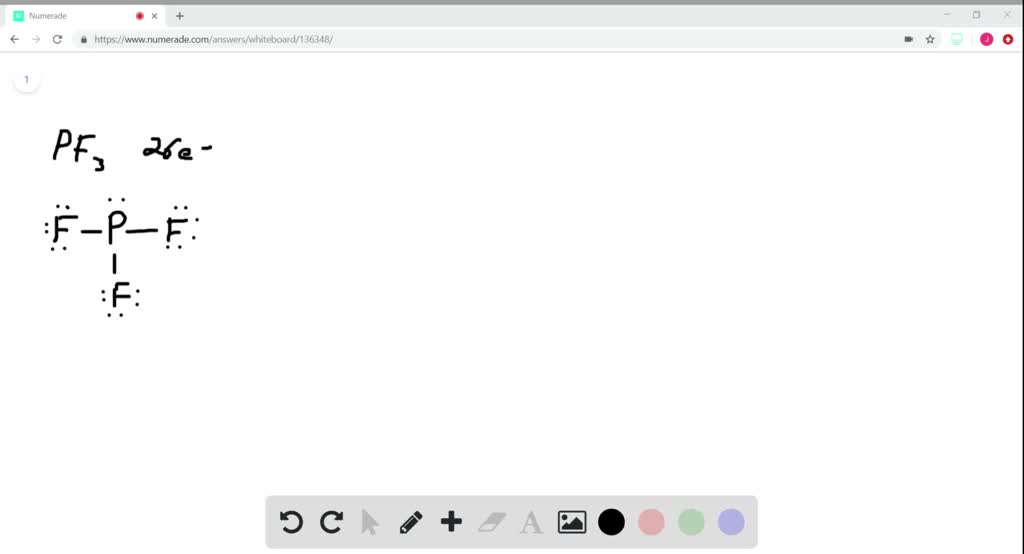
SOLVED(a) Draw the dominant Lewis structure for the phosphorus

draw the lewis structure for pf3 including lone pairs 160vanbruntstreet
Web The P−F Bond In Pf3 Is 109.5∗ The Molecule Pf3 Is 120∗ <109.5∘ 180∘ Your Solution’s Ready To Go!
Web Lewis Structure Of Pf3 Contains Three Single Bonds Between The Phosphorus (P) Atom And Each Fluorine (F) Atom.
Web A Lewis Diagram Shows How The Valence Electrons Are Distributed Around The Atoms In A Molecule.
The Phosphorus Atom (P) Is At The Center And It Is Surrounded.
Related Post: