Draw The Electron Configuration For A Neutral Atom Of Neon
Draw The Electron Configuration For A Neutral Atom Of Neon - Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. An electron configuration shows the distribution of electrons of an atom or a molecule. Neon is the tenth element with a total of 10 electrons. Web to write the electron configuration for neon, the first two electrons enter the 1s orbital, the next two electrons enter the 2s orbital, and the remaining six electrons enter the 2p. Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the. Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. Web to determine the electron configuration for any particular atom, we can “build” the structures in the order of atomic numbers. Determine the electron configuration of ions. The n=1 shell can only hold 2 electrons, so the remaining 8. An electron configuration diagram is a. Then, add or remove electrons depending on the ion's charge. An electron configuration shows the distribution of electrons of an atom or a molecule. Web how to write the electron configuration for neon. The atomic number of p is 15. An electron configuration diagram is a. Web the important thing to know for these diagrams is that the first energy level can hold 2 electrons, the second energy level can hold 8 electrons and the third energy level can. Determine the electron configuration of ions. Web neon has atomic number 10, so a neon atom has 10 protons in its nucleus and therefore 10 electrons. By. What is an electron configuration? Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Web for hydrogen, therefore, the single electron is placed in the 1s orbital, which is the orbital lowest in energy (figure 6.29), and the electron configuration is written. Beginning with hydrogen, and continuing.. Web neon has atomic number 10, so a neon atom has 10 protons in its nucleus and therefore 10 electrons. What is an electron configuration? Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. Web electron configuration chart of all elements is mentioned in the table below. In writing the electron configuration. Beginning with hydrogen, and continuing. Then, add or remove electrons depending on the ion's charge. The shorthand electron configuration (or noble gas configuration) as well as. Web electron configuration chart of all elements is mentioned in the table below. The atomic number of p is 15. Web how to write the electron configuration for neon. Determine the electron configuration of ions. Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the. The atomic number of p is 15. Beginning with hydrogen, and continuing. Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Determine the electron configuration of ions. Then, add or remove electrons depending on the ion's charge. Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. A neon atom is a neutral atom that has 10 atomic numbers which imply it has a total of 10 electrons. Beginning with hydrogen, and continuing. An electron configuration shows the distribution of electrons of an atom or a molecule. Justify the observed. Web to find the electron configuration for an ion, first identify the configuration for the neutral atom. In writing the electron configuration for neon the first two electrons will go. Web to determine the electron configuration for any particular atom, we can “build” the structures in the order of atomic numbers. Web the important thing to know for these diagrams. Web for hydrogen, therefore, the single electron is placed in the 1s orbital, which is the orbital lowest in energy (figure 6.29), and the electron configuration is written. In writing the electron configuration for neon the first two electrons will go. Web to write the electron configuration for neon, the first two electrons enter the 1s orbital, the next two. Web neon electron configuration using the aufbau principle. Web to determine the electron configuration for any particular atom, we can “build” the structures in the order of atomic numbers. Then, add or remove electrons depending on the ion's charge. Web to find the electron configuration for an ion, first identify the configuration for the neutral atom. Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the. Web electron configuration chart of all elements is mentioned in the table below. An electron configuration diagram is a. Web atoms use their electrons to participate in chemical reactions, so knowing an element’s electron configuration allows you to predict its reactivity—whether, and how, it will. By knowing the electron configuration of an element, we can predict and explain. Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. Web for hydrogen, therefore, the single electron is placed in the 1s orbital, which is the orbital lowest in energy (figure 6.29), and the electron configuration is written. Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: The n=1 shell can only hold 2 electrons, so the remaining 8. The atomic number of p is 15. Beginning with hydrogen, and continuing. Neon is the tenth element with a total of 10 electrons.
PPT Orbital Filling Electron Configurations PowerPoint Presentation

Neon(Ne) electron configuration and orbital diagram
![[5 Steps] Electron Configuration for or of Neon in Just 5 Steps](https://2.bp.blogspot.com/-jMs9FCVMeSw/XD4FtZiSVlI/AAAAAAAAYZg/rJnlLKM6S6EHv8FmJZr4pj2PmzTZjk0hQCLcBGAs/s1600/20190115_220744.jpg)
[5 Steps] Electron Configuration for or of Neon in Just 5 Steps

Diagram representation element neon Royalty Free Vector
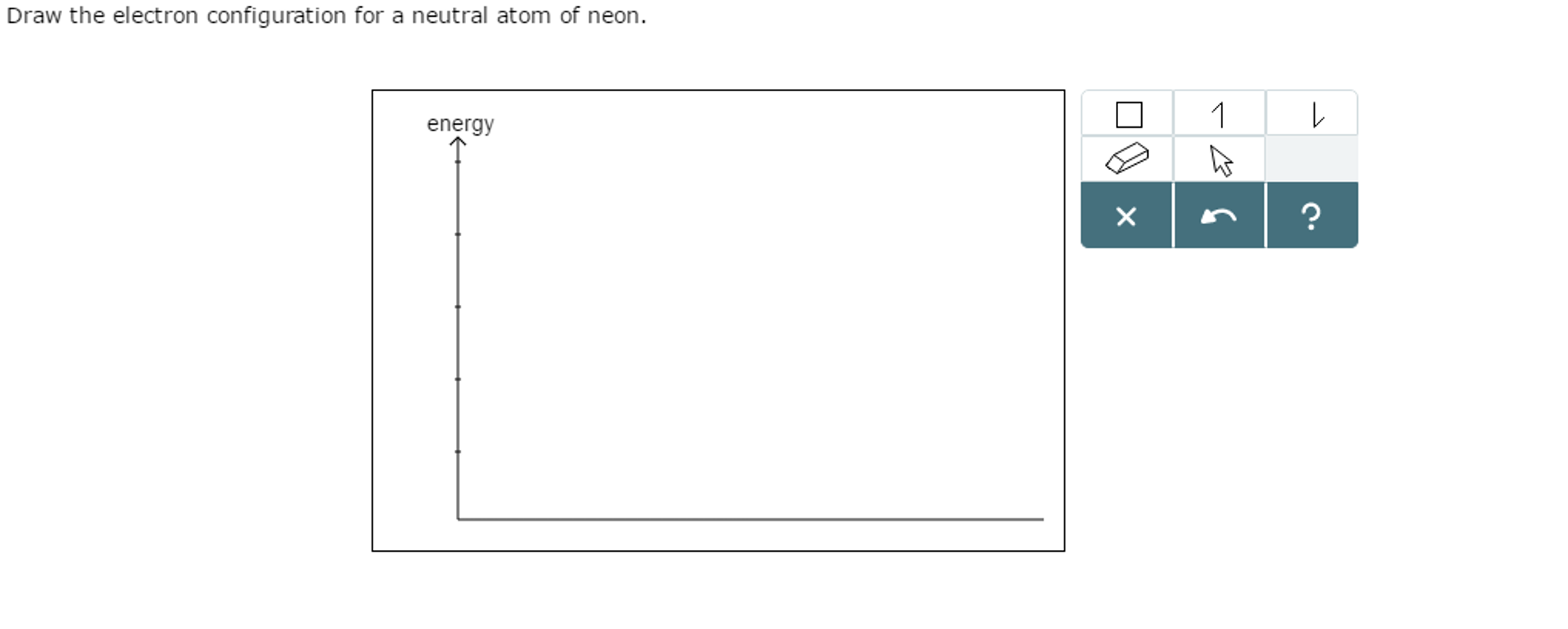
Solved Draw the electron configuration for a neutral atom of
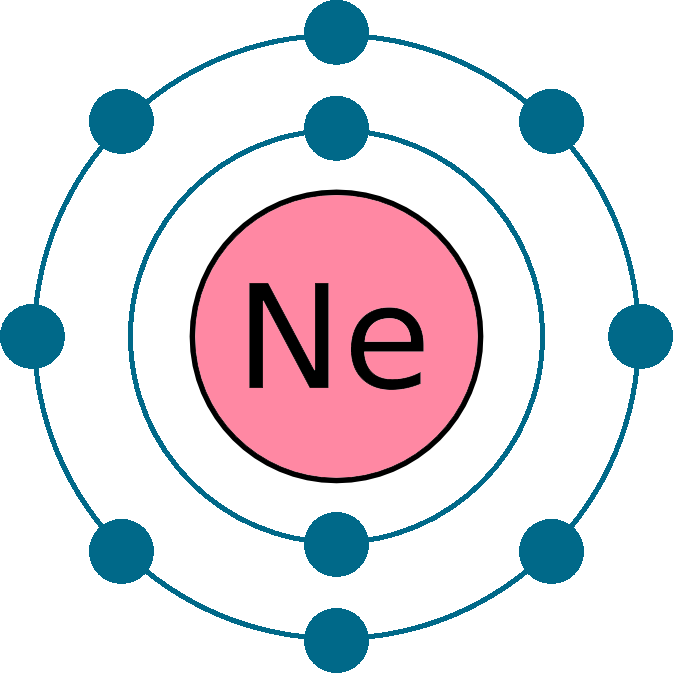
Neon Element (Ne 10) of Periodic Table Periodic Table FlashCard
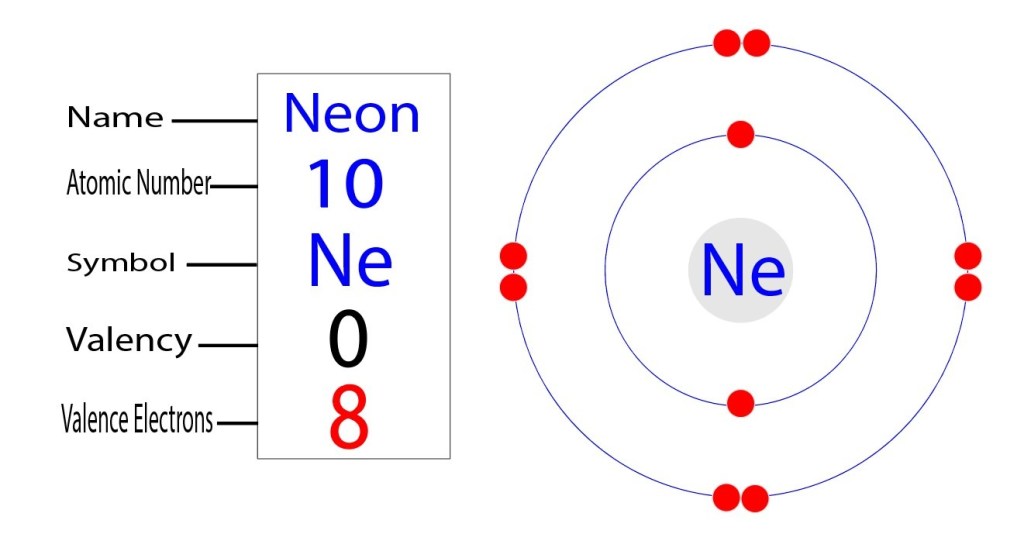
Orbital Diagram For Neon (Ne) Neon Electron Configuration
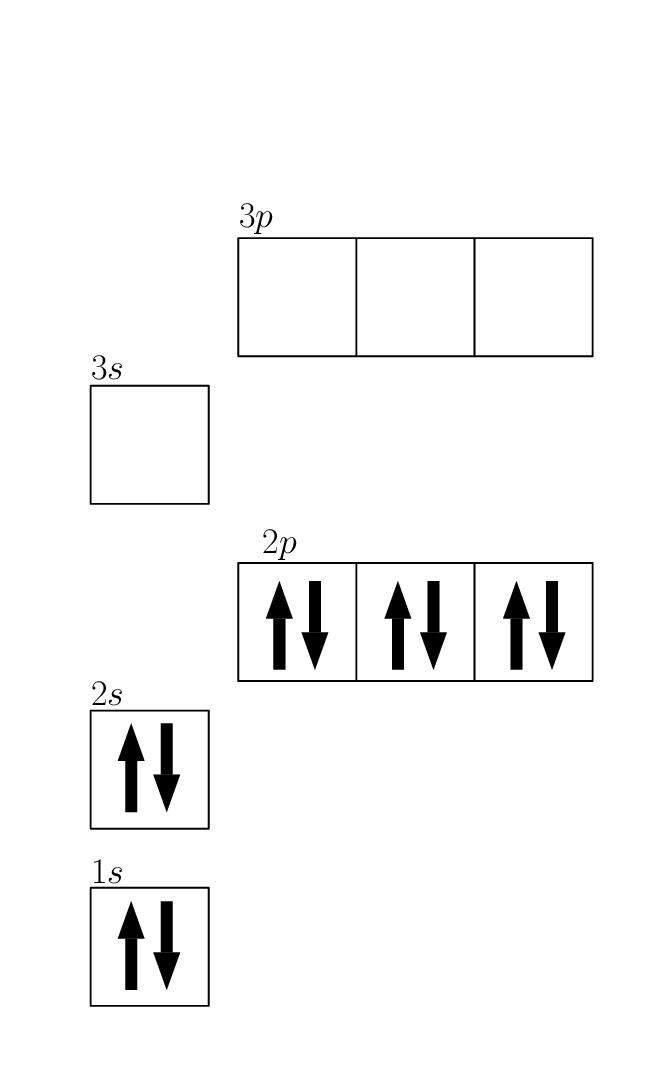
Orbital Diagram For Neon

Neon Orbital Notation
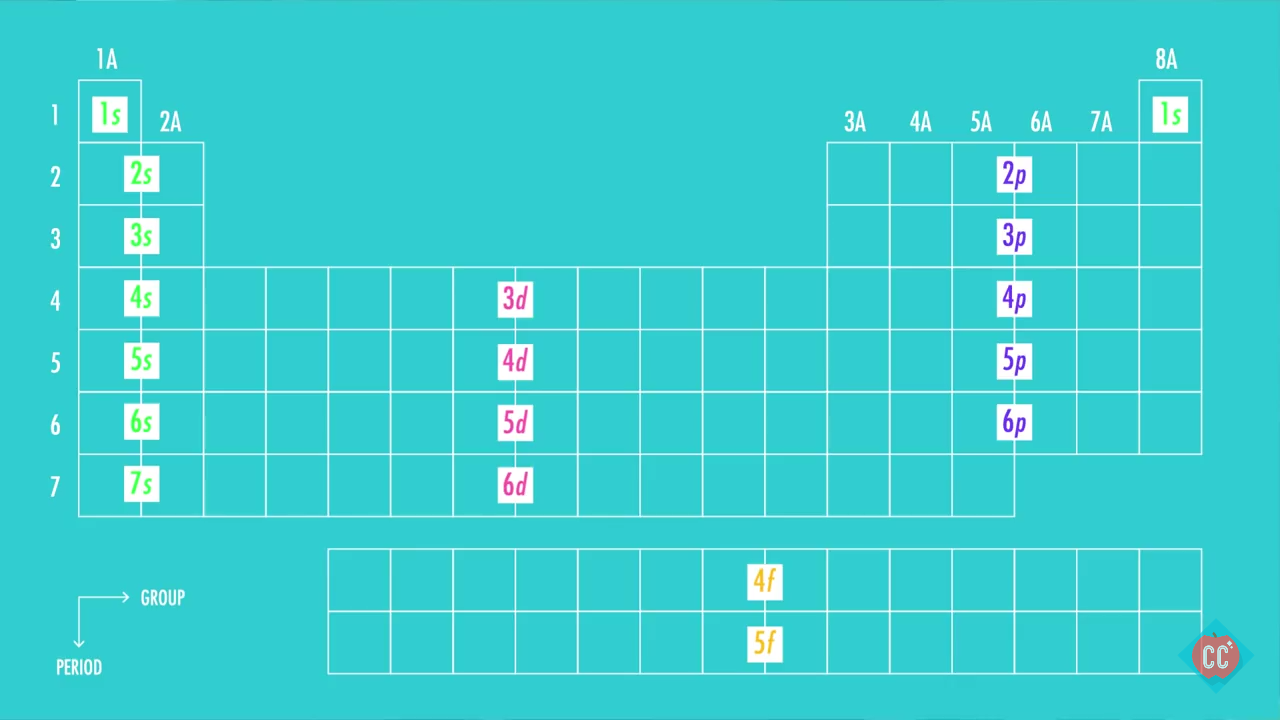
What is the groundstate electron configuration of a neutral atom of
Web Neon Has Atomic Number 10, So A Neon Atom Has 10 Protons In Its Nucleus And Therefore 10 Electrons.
Web Using Figure \(\Pageindex{2}\) As Your Guide, Write The Electron Configuration Of A Neutral Phosphorus Atom.
Web To Write The Electron Configuration For Neon, The First Two Electrons Enter The 1S Orbital, The Next Two Electrons Enter The 2S Orbital, And The Remaining Six Electrons Enter The 2P.
Determine The Electron Configuration Of Ions.
Related Post: