Draw The Electron Configuration For A Neutral Atom Of Helium
Draw The Electron Configuration For A Neutral Atom Of Helium - Web atoms use their electrons to participate in chemical reactions, so knowing an element’s electron configuration allows you to predict its reactivity—whether, and how, it will interact with atoms of other elements. From the pauli exclusion principle, we know that an orbital can contain two electrons with opposite spin, so we place the second electron in the same orbital as the first but pointing down. Web a neutral helium atom, with an atomic number of 2 (z = 2), has two electrons. 25 + 111 show transcribed image text there are 2 steps to solve this one. It denotes a full s orbital. The element atomic number and name are listed in the upper left. Web the electron configuration for helium shows a full outer shell and is helium is therefore called a nobel gas. Draw the electron configuration for a neutral atom of helium. This means that the electron configuration for helium has to account for only #2# electrons. Want to join the conversation? Web electron configuration chart of all elements is mentioned in the table below. Web atoms use their electrons to participate in chemical reactions, so knowing an element’s electron configuration allows you to predict its reactivity—whether, and how, it will interact with atoms of other elements. The number of the principal quantum shell, n, the letter that designates the orbital type. The aufbau principle is most useful for the first 20 elements: Want to join the conversation? Web this is the electron configuration of helium;. A neutral helium atom will thus have #2# electrons surrounding its nucleus. 2 electrons are present in s shell and is complete configuration in ground state. Draw the electron configuration for a neutral atom of helium. Web this is the electron configuration of helium;. Web find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. The n = 1 shell is completely filled in a helium atom. It denotes a full s orbital. We place one electron in the orbital that is lowest in energy, the 1 s orbital. From the pauli exclusion principle, we know that an orbital can contain two electrons with opposite spin, so we place the second electron in the same orbital as the first but pointing down. Web the electron configuration for helium shows a full outer shell. The aufbau principle is most useful for the first 20 elements: Energy 1 i x $ ? The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the table. The n = 1 shell is completely filled in a helium atom. The n = 1 shell is completely filled in a helium. Energy your solution’s ready to go! Web the electron configuration and orbital diagram of helium are: Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Your solution’s ready to go! This is the electron configuration of helium; The next atom is the alkali metal lithium with an atomic number of 3. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Want to join the conversation? Predict the charge of common metallic and nonmetallic elements, and write their electron configurations. The. The next atom is the alkali metal lithium with an atomic number of 3. From the pauli exclusion principle, we know that an orbital can contain two electrons with opposite spin, so we place the second electron in the same orbital as the first but pointing down. Web the electron configuration and orbital diagram of helium are: Web a neutral. The n = 1 shell is completely filled in a helium atom. The next atom is the alkali metal lithium with an atomic number of 3. Energy your solution’s ready to go! Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. This means that the electron configuration for helium has. Both electrons fit into the 1 s subshell because s subshells contain one s orbital which can hold up to 2 electrons; The n = 1 shell is completely filled in a helium atom. The next atom is the alkali metal lithium with an atomic number of 3. Web helium atoms have 2 electrons. Web to write the electron configuration. Draw the electron configuration for a neutral atom of helium. Your solution’s ready to go! A neutral helium atom will thus have #2# electrons surrounding its nucleus. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the table. The next atom is the alkali metal lithium with an atomic number of 3. Web helium atoms have 2 electrons. The n = 1 shell is completely filled in a helium atom. Web the electron configuration and orbital diagram of helium are: The upper right side shows the number of electrons in a neutral atom. 2 electrons are present in s shell and is complete configuration in ground state. Both electrons fit into the 1 s subshell because s subshells contain one s orbital which can hold up to 2 electrons; The element atomic number and name are listed in the upper left. The n = 1 shell is completely filled in a helium atom. Web the final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. This is the electron configuration of helium; Web a neutral helium atom, with an atomic number of 2 (z = 2), has two electrons.
Helium atom Plugon
Electron Arrangement in Atoms CK12 Foundation
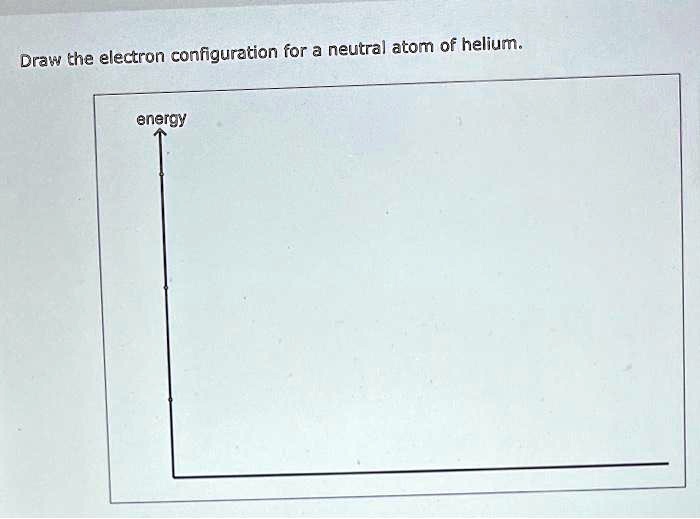
SOLVED Draw the electron configuration for a neutral atom of helium
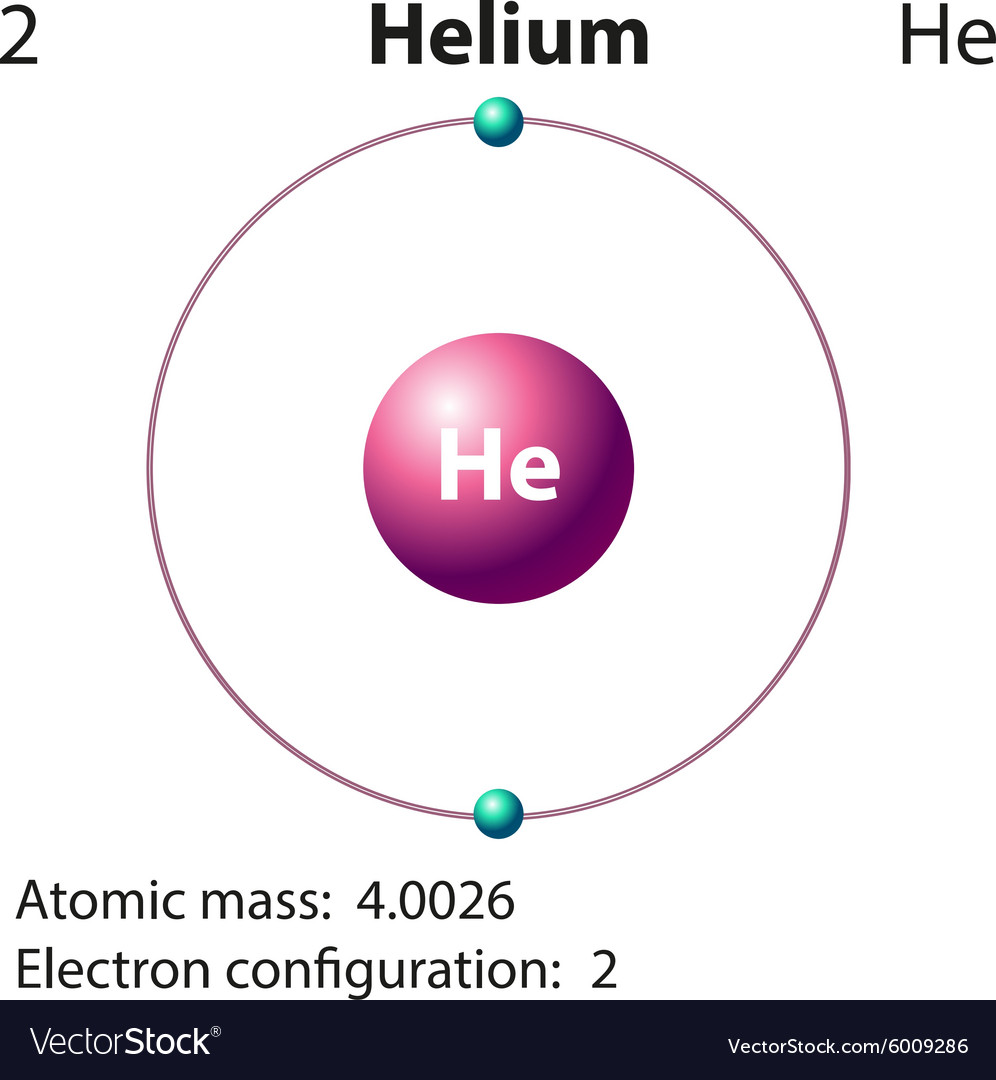
Diagram representation of the element helium Vector Image

Draw The Electron Configuration For A Neutral Atom Of Helium. Drawing

Helium Electron Configuration Electron configuration, Electrons
Helium Atom Drawing at GetDrawings Free download
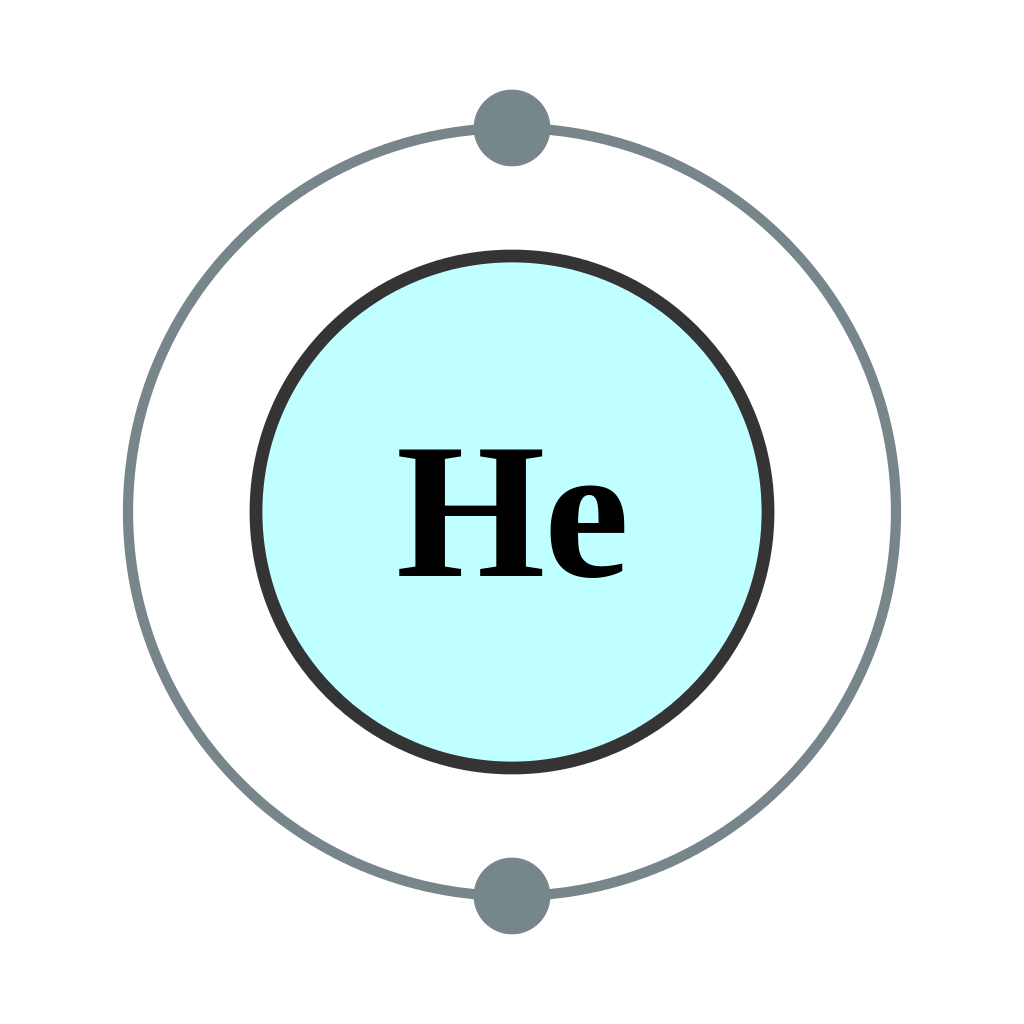
How To Find the Helium Electron Configuration (He)
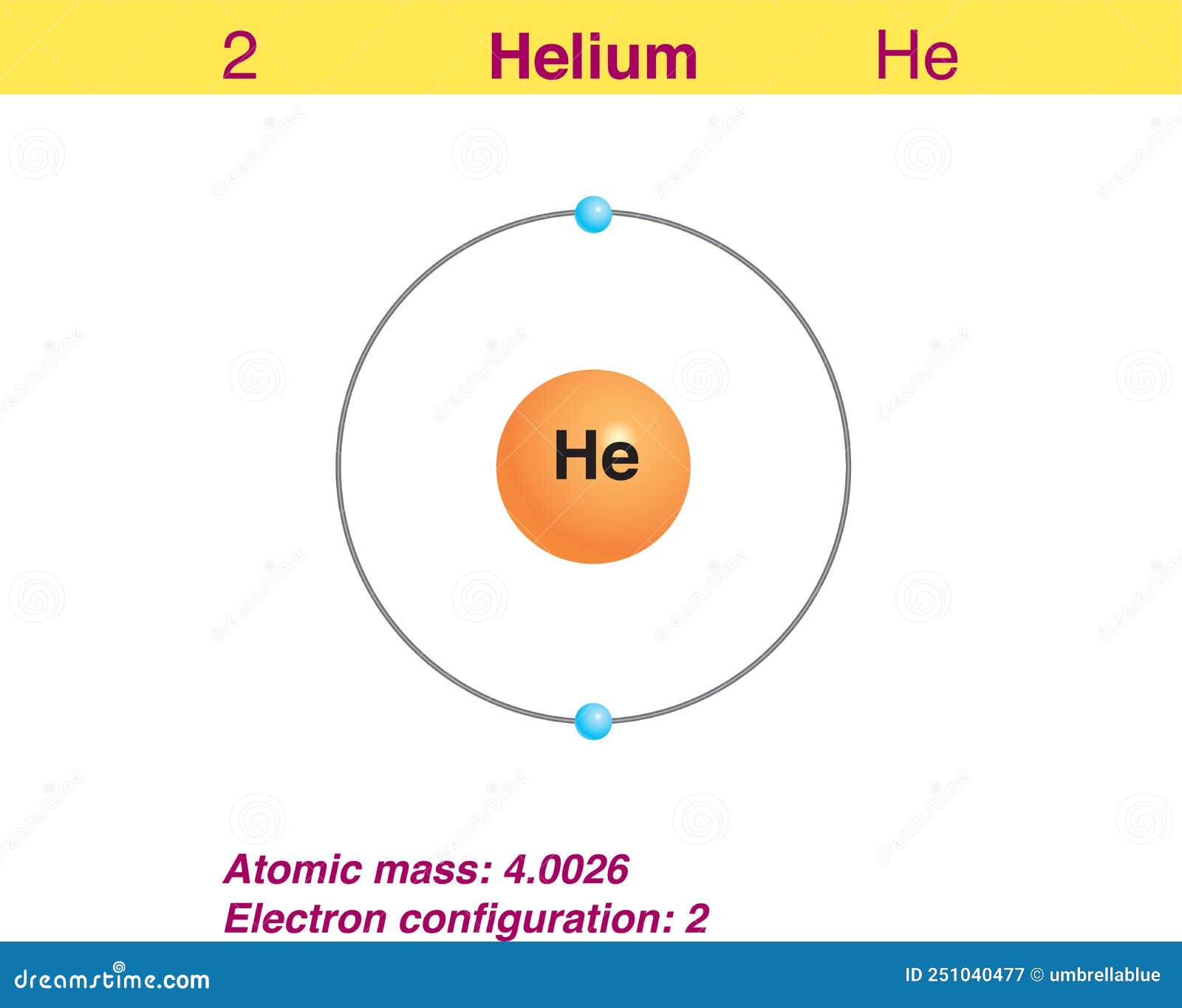
Electron of the Element Helium Stock Vector Illustration of electron
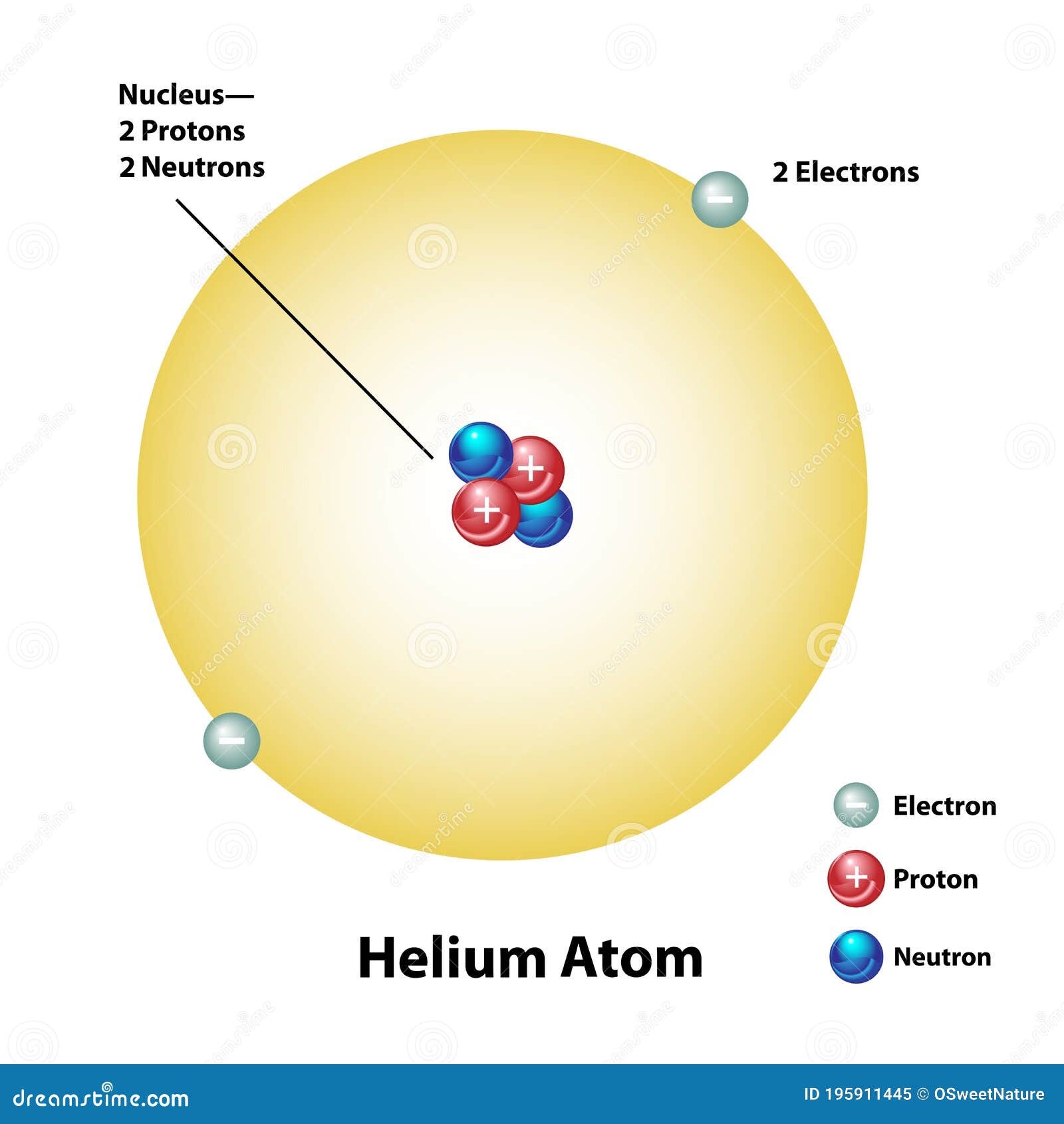
Helium Atom with Nucleus and Electron Shell Stock Vector Illustration
How To Write The Electron Configuration For Helium.
Web Electron Configuration Chart Of All Elements Is Mentioned In The Table Below.
Identify And Explain Exceptions To Predicted Electron Configurations For Atoms And Ions.
The Aufbau Principle Is Most Useful For The First 20 Elements:
Related Post:
