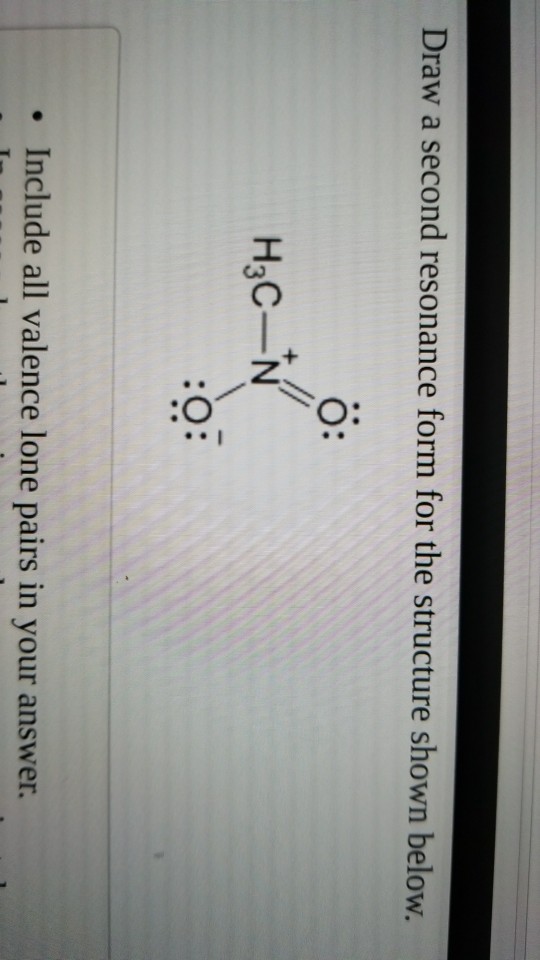
Draw A Second Resonance Form For The Structure Shown Below
Draw A Second Resonance Form For The Structure Shown Below - In this case, we have a nitrogen atom with a lone pair and a carbon atom with a double bond. This is where we can see the structure. The lone pair will form a negative This is what the system looks like. Web draw the resonance structures of molecules or ions that exhibit delocalization. We can say that this is a compound here, or this is a double bond oxygen, or this is a lone pair. I hopped the neighboring double bond onto the carbon and turned it into a double bond. We need to match each linear system with a face plane direction. H3c nh2 your solution’s ready to go! Web identify the positions of the atoms and the location of the pi bonds and lone pairs in the given structure to determine which electrons can be delocalized in creating a new resonance form. If we see that compound, which is here, we can say that this is a thing here here, or this is the oxygen here, and this is a long pair like this,… We can say that this is a compound here, or this is a double bond oxygen, or this is a lone pair. Equivalent lewis structures are called resonance. I can do this pattern for residents. Web draw the major resonance contributor of the structure below. If we see that compound, we can say that this is a thing here, or this is a long pair like this, and it is a double bond oxygen, and here this is a lone pair. • in cases where there is more. We can see the resonal structure here. Web identify the positions of the atoms and the location of the pi bonds and lone pairs in the given structure to determine which electrons can be delocalized in creating a new resonance form. Ö+ ch3 draw a second resonance form for the structure shown below. The lone pair will form a negative. Here we can say the resonance Web draw the major resonance contributor of the structure below. We can convert one resonance form into another by showing the movement of electrons between bonds and lone pairs (or vice versa). Explain why your contributor is the major one. Combine the resonance structures by adding (dotted) bonds where other resonance bonds can be. They are used when there is more than one way to place double bonds and lone pairs on atoms. We can say that this is a compound here, or this is a double bond oxygen, or this is a lone pair. Web draw the resonance structures of molecules or ions that exhibit delocalization. We just need a graphical tool to. Web draw a second resonance form for the structure shown below. Web first, we need to identify the atoms that can move their electrons to form a double bond or a lone pair. Web the second resonance structure of ozone is very similar, as it has one positively charged oxygen, one negatively charged oxygen, and one neutral oxygen with two. • in cases where there is more than one answer, just draw one. Use the concept of resonance to explain structural features of molecules and ions. We need to match each linear system with a face plane direction. If we see that compound, we can say that this is a thing here, or this is a long pair like this,. Combine the resonance structures by adding (dotted) bonds where other resonance bonds can be formed. Web draw the major resonance contributor of the structure below. Include in your figure the appropriate curved arrows showing how you got from the given structure to your structure. I hopped the neighboring double bond onto the carbon and turned it into a double bond.. Use the concept of resonance to explain structural features of molecules and ions. A negative charge will be formed when this lone pair arrives here. If you've ever had a negative charge next to a double bond, i don't think you should. The lone pair will form a negative Web draw the resonance structures of molecules or ions that exhibit. Use the concept of resonance to explain structural features of molecules and ions. Web the second resonance structure of ozone is very similar, as it has one positively charged oxygen, one negatively charged oxygen, and one neutral oxygen with two bonds again. Determine the relative stability of resonance structures using a set of rules. Web draw the resonance structures of. Move one of the lone pairs from the neighboring atom to form a double bond with the atom that initially had a double bond. H ch3 h3c n ch3 • include all valence lone pairs in your answer. Web draw the resonance structures of molecules or ions that exhibit delocalization. Determine the relative stability of resonance structures using a set of rules. We will try to find out what our equation is. Web introducing curved arrows, a tool for showing the movement of electrons between resonance structures. Web first, we need to identify the atoms that can move their electrons to form a double bond or a lone pair. Identify the molecule with a double bond and a neighboring atom with a lone pair of electrons. However, in this structure, the right oxygen atom has the negative charge and the left oxygen atom is neutral. Explain why your contributor is the major one. Here we can say the resonance This is what the system looks like. Web draw the resonance structures of molecules or ions that exhibit delocalization. We need to match each linear system with a face plane direction. Sometimes one dot structures is not enough to completely describe a molecule or an ion, sometimes you need two or more, and here's an example: If you've ever had a negative charge next to a double bond, i don't think you should.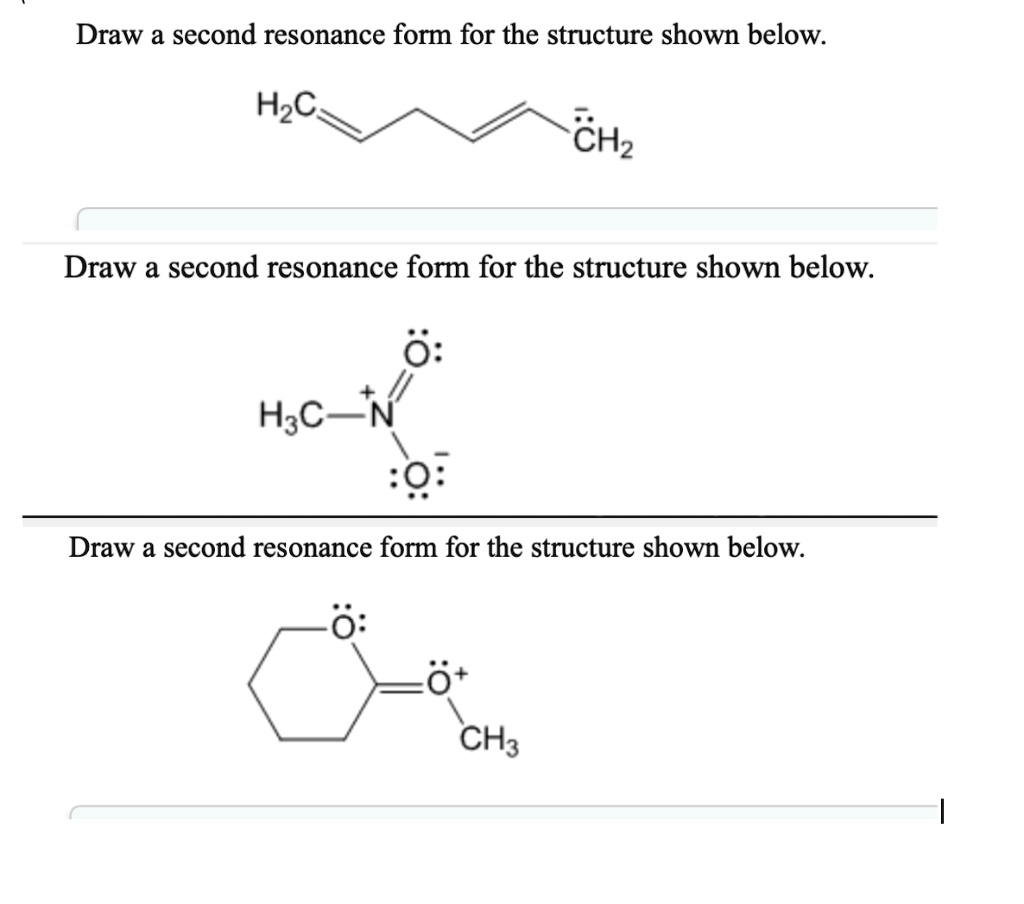
SOLVED Draw a second resonance form for the structure shown below H3C
Solved Draw a second resonance form for the structure shown
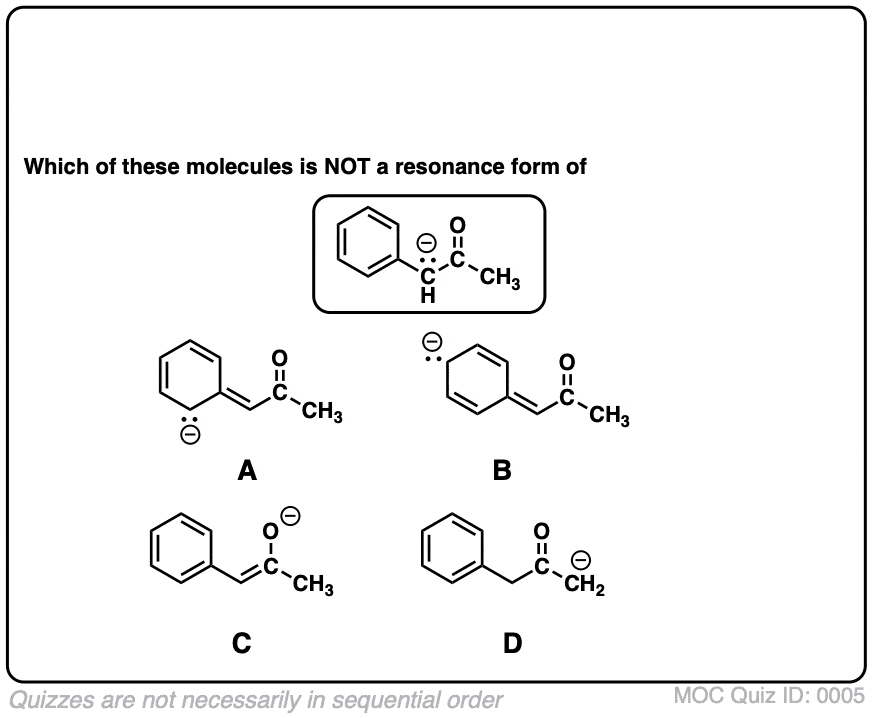
Resonance Structures Practice Master Organic Chemistry
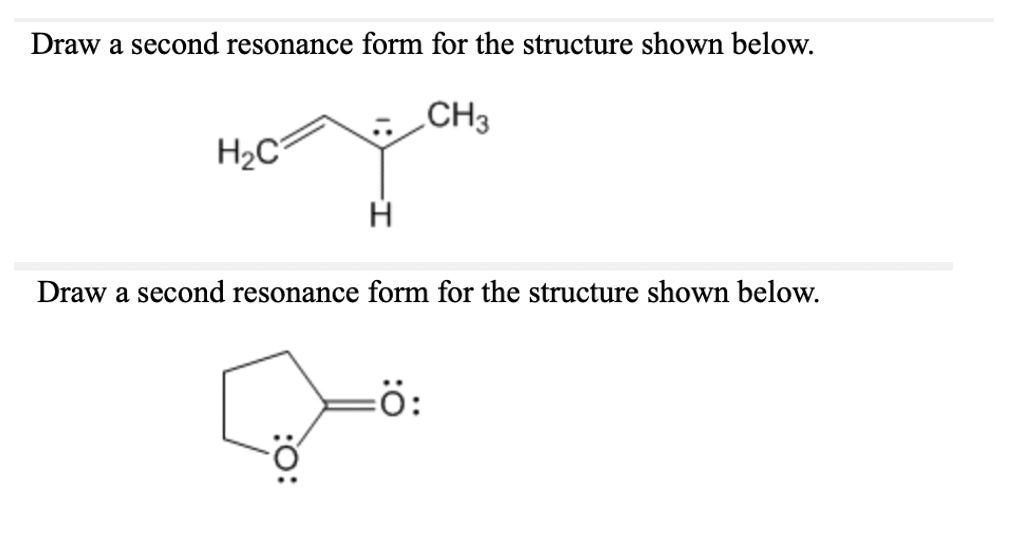
SOLVED Draw a second resonance form for the structure shown below CH3
[Solved] Draw a second resonance form for the structure shown below. H
[Solved] Draw a second resonance form for the structures shown below

Resonance Structures Easy Hard Science
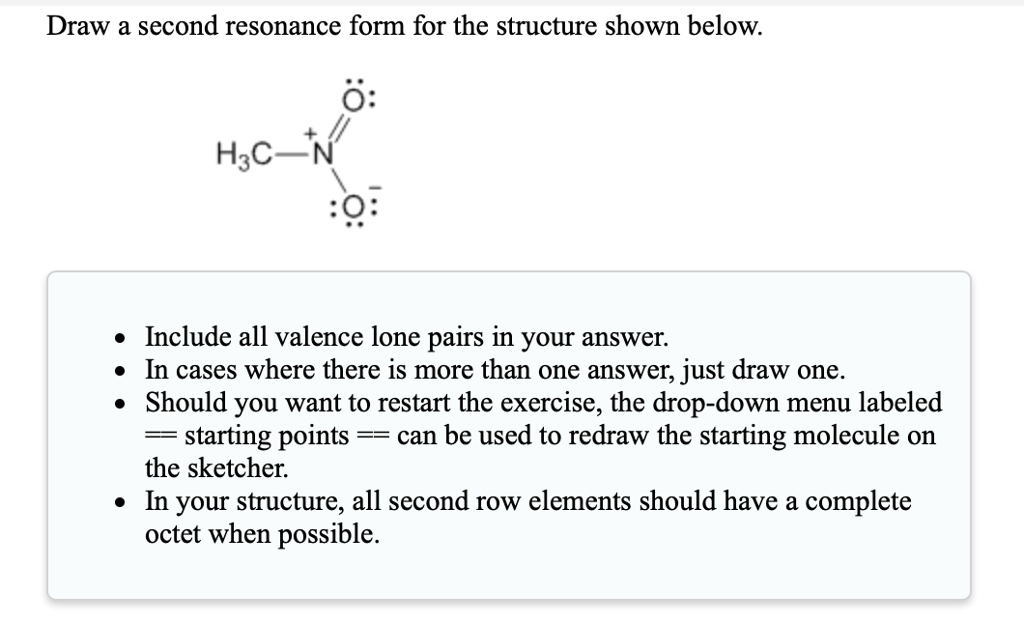
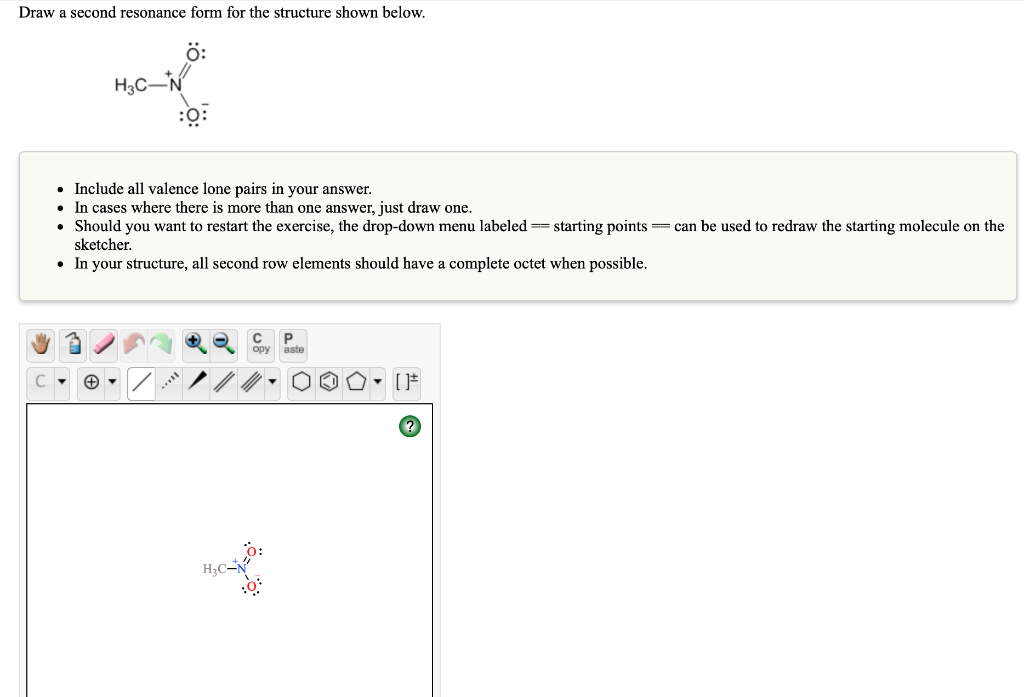
SOLVEDDraw a second resonance form for the structure shown below 0
Solved Draw a second resonance form for the structure shown

Solved Draw a second resonance form for the structure shown
We Can Convert One Resonance Form Into Another By Showing The Movement Of Electrons Between Bonds And Lone Pairs (Or Vice Versa).
I Can Do This Pattern For Residents.
In This Case, We Have A Nitrogen Atom With A Lone Pair And A Carbon Atom With A Double Bond.
Include In Your Figure The Appropriate Curved Arrows Showing How You Got From The Given Structure To Your Structure.
Related Post: