Draw A Lewis Structure For Co
Draw A Lewis Structure For Co - Web lewis structure of co (or carbon monoxide) contains one triple bond between the carbon (c) atom and oxygen (o) atom. Web in this video you’ll learn how to draw lewis dot structures for covalent compounds. Web the lewis structure for co has 10 valence electrons. Step method to draw the lewis structure of co. In addition to this, the contribution of the lone pair of electrons further contributes to the linear geometry of the carbon monoxide (co) molecule. So we have a carbon and an oxygen atom bonded together. Web all the oxidation states must add up to 0 for a neutral molecule. Drawing lewis structures for bf3, pf3 and brf3; Draw a trial structure by putting electron pairs around every atom until each gets an octet. The outer (valence) shell configuration of carbon and oxygen atoms are: This is the co lewis structure: That will normally be the least electronegative atom (c). See the big list of lewis structures. Web the lewis structure (lewis dot diagram) for co. Web how to draw the lewis dot structure for carbon monoxide. That will normally be the least electronegative atom (c). Web the lewis structure of carbon monoxide (co) has a triple bond formation where one is strong sigma, and the other two are weak pi bonds. We have 4 valence electrons for carbon and 6 for oxygen, for a total of 10 valence electrons. The carbon atom and oxygen atom, both. Put one electron pair in each bond 4. Web co is a gas used primarily in chemical synthesis, metal production, as a fuel and as a chemical catalyst. Web what is the lewis structure of carbon monoxide co? (there are two pi bonds and one sigma bond in a triple bond, one sigma and one pi bond in a double. The video covers the basic lewis structures you'll see in an introductor. 112.8 pm, bond energy around 1072 kj/mol. Drawing lewis structures for bf3, pf3 and brf3; The carbon atom and oxygen atom, both have one lone pair. Where there are electron pairs, construct one bond line for each electron pair. Then determine how many valence electrons each element has: Put one electron pair in each bond 4. Valence electrons count the total number of valence electrons of carbon and oxygen atom. Web co is a gas used primarily in chemical synthesis, metal production, as a fuel and as a chemical catalyst. That will normally be the least electronegative atom (c). See the big list of lewis structures. Web the lewis structure for co (carbon monoxide) is a crucial component of understanding its molecular properties and reactivity. Valence electrons count the total number of valence electrons of carbon and oxygen atom. Web the lewis structure for co has 10 valence electrons. Breaking the octet rule ; In total, there are 10 valence electrons. Web lewis structure of carbon monoxide (co) molecule. Draw a trial structure by putting electron pairs around every atom until each gets an octet. Fill outer atoms with electrons 5. Where there are electron pairs, construct one bond line for each electron pair. See the big list of lewis structures. Drawing lewis structures for bf3, pf3 and brf3; Connect the atoms with single bonds; Web what is the lewis structure of carbon monoxide co? Web to draw the lewis structure of co, it is vital to know the total number of valence electrons of the molecule. So we have a carbon and an oxygen atom bonded together. How to draw the lewis dot structure for carbon monoxide; Valence electrons count the total number of valence electrons of carbon and oxygen atom. Web lewis structure of carbon monoxide (co) molecule. Triple bond between c (sp hybridized) and o (sp^2 hybridized), 10 valence electrons total. Web i quickly take you through how to draw the lewis structure of carbon monoxide. Check the octet rule (formal. In total, there are 10 valence electrons. Web how to draw the lewis dot structure for carbon monoxide. (there are two pi bonds and one sigma bond in a triple bond, one sigma and one pi bond in a double. (there are two pi bonds and one sigma bond in a triple bond, one sigma and one pi bond in a double bond, and one sigma bond in. Triple bond between c (sp hybridized) and o (sp^2 hybridized), 10 valence electrons total. For the co lewis structure you'll need a triple bond between the carbon and oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the co molecule. Web the lewis structure of carbon monoxide (co) has a triple bond formation where one is strong sigma, and the other two are weak pi bonds. Web i quickly take you through how to draw the lewis structure of carbon monoxide. In this article, we will explore how to draw the lewis structure for co, and the significance of co in various fields of chemistry. We will first find out the valence electrons for both these atoms and then add both these valence electrons to get the total valence electrons of co. Web co lewis structure: The valence electrons available are 4+6=10. Web how to draw the lewis dot structure for carbon monoxide. In next sections, we will draw co lewis structure step by step. Web the lewis structure (lewis dot diagram) for co. In the lewis structure of carbon monoxide, both atoms have eight electrons in their valence shells. Where there are electron pairs, construct one bond line for each electron pair. Web in this video you’ll learn how to draw lewis dot structures for covalent compounds. For the co structure use the periodic table to find the total number of valence electrons for the co.
Carbon Monoxide Lewis Structure
Co Molecule Lewis Structure

Co Molecule Lewis Structure
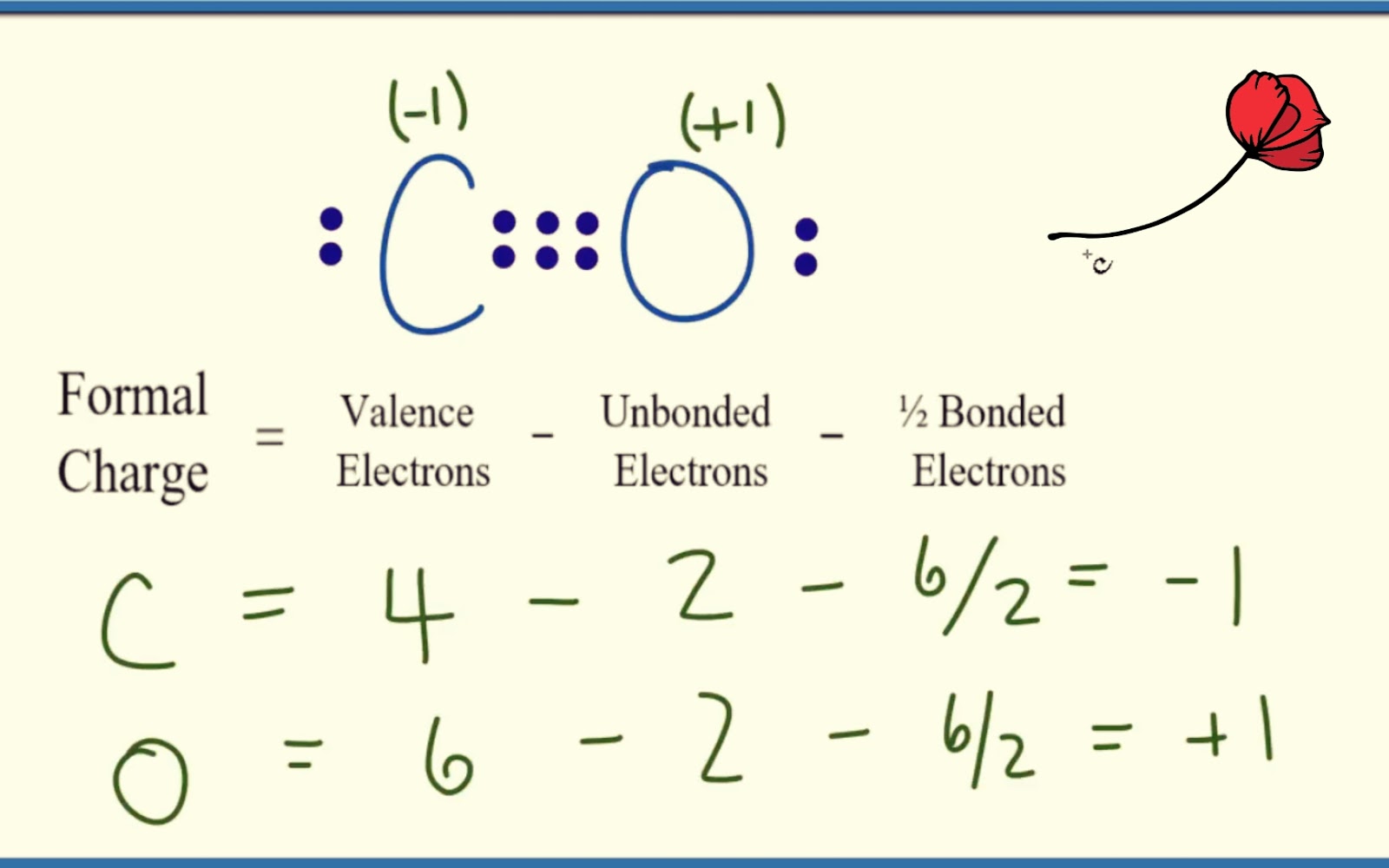
CO Lewis Structure ,Valence Electrons ,Formal Charge ,Polar or Nonpolar

Draw The Lewis Structure Of Co

CO Lewis Structure, Geometry, and Hybridization Techiescientist

Lewis Structure of CO (Carbon Monoxide) YouTube

How do you draw the Lewis structure for CO (Carbon monoxide)?Write
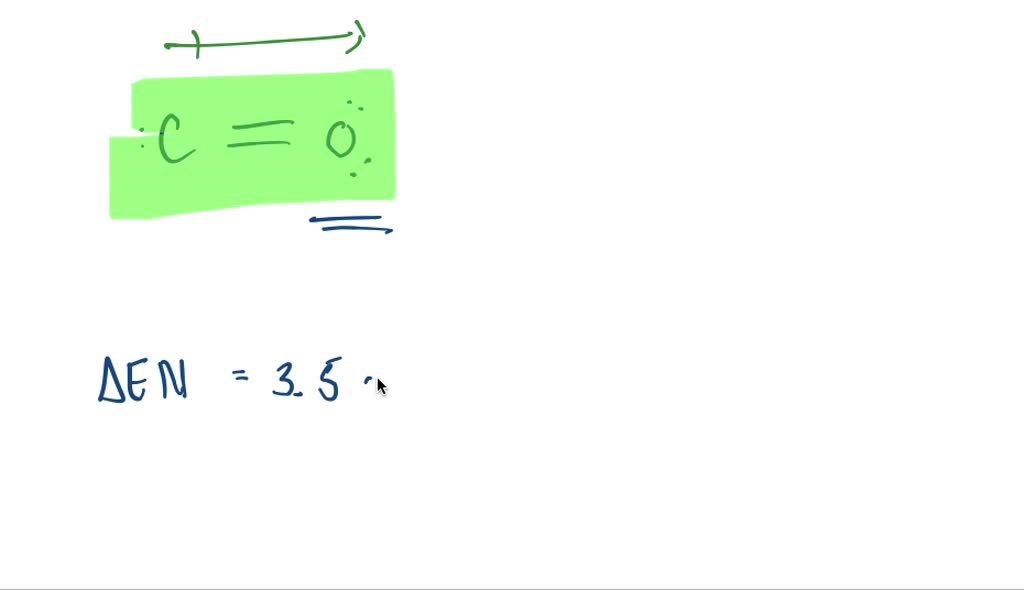
SOLVEDDraw the Lewis structure for CO with an arrow representing the
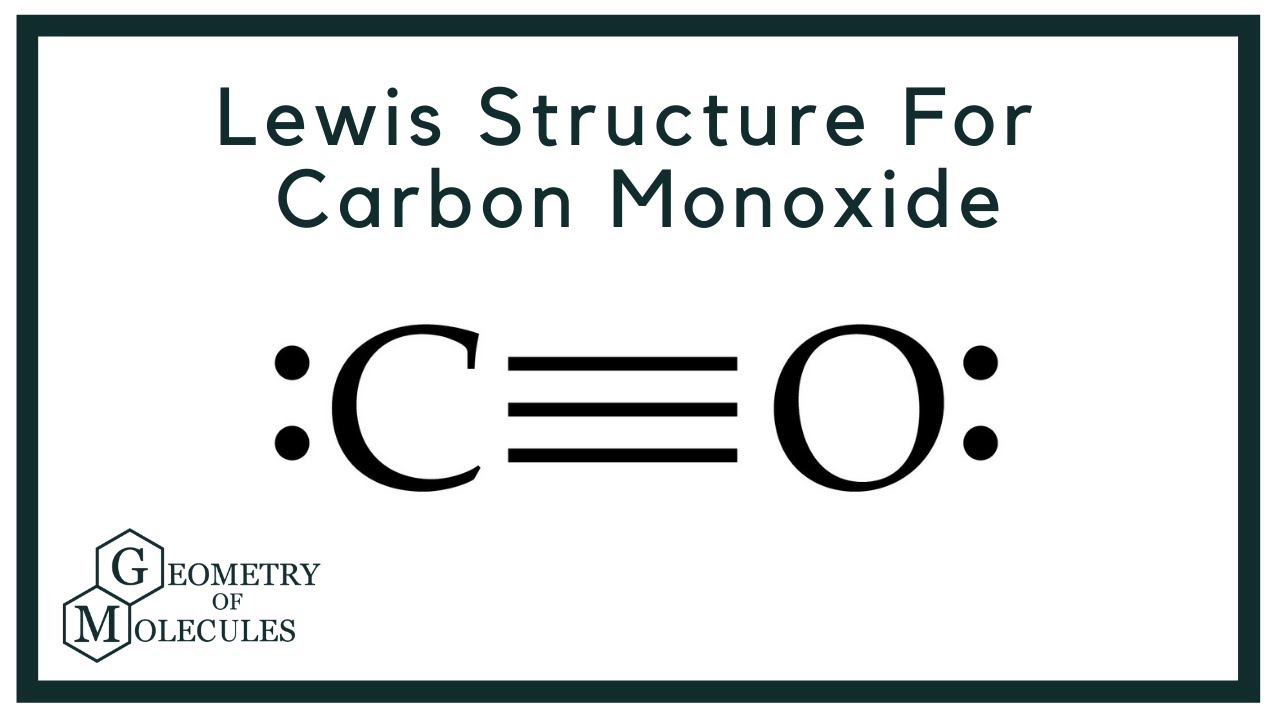
Lewis Structure for CO (Carbon Monoxide) YouTube
Connect The Atoms With Single Bonds;
Web Lewis Structure Of Carbon Monoxide (Co) Molecule.
Valence Electrons Count The Total Number Of Valence Electrons Of Carbon And Oxygen Atom.
Web Drawing Lewis Structures For Molecules With One Central Atom:
Related Post: