Design Transfer
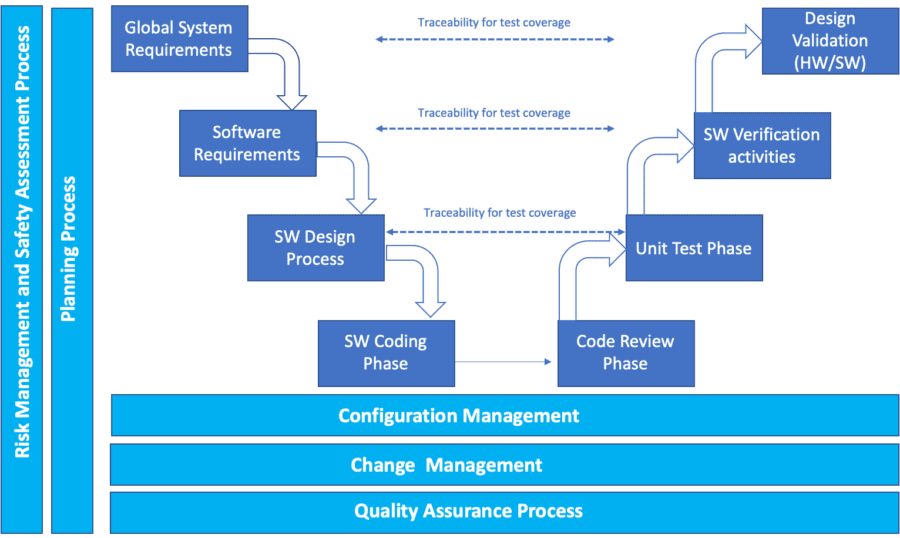
Design Transfer - 2016 say about design transfer? Web the design transfer process includes a number of activities—like demonstrating successful design verification and validation and ensuring your device master record (dmr) is complete and accurate—that must occur before manufacturing can begin. Take these points into consideration to enable a smooth design transfer. Web moving from medical device design to production can be a challenging process. And because of this, the process of transferring a medical device from product development to production (often referred to as “design transfer”) begins during design validation. Design transfer is the culmination of the medical device design team’s efforts during which the product and process designs are transferred to production. Web what is design transfer? Web the concepts, implementation, and timing of major design transfer associated tasks (design verification, process validation, design validation, design outputs, and dmr) can sometimes be best managed and understood using process flow diagrams and charts, as illustrated in figure 1. Design transfer is a component of the fda’s medical device quality system regulation design controls. Web design transfer is a pivotal phase that bridges the gap between concept and reality in medical device development. Web of these transitions, design transfer, the introduction of a design to production, may be the most important. 2016 say about design transfer? How can you be sure no one in your supply chain drops the ball as they assume control of the product you’ve taken so long to design and develop? Design transfer is the culmination of the medical. Web moving from medical device design to production can be a challenging process. Web design transfer is a pivotal phase that bridges the gap between concept and reality in medical device development. 2016 say about design transfer? Web design transfer begins. Effective design transfer requires a thorough assessment of the product's design documentation, selection of components, and careful definition of. 5/5 (29k reviews) Web design transfer is a pivotal phase that bridges the gap between concept and reality in medical device development. Web the concepts, implementation, and timing of major design transfer associated tasks (design verification, process validation, design validation, design outputs, and dmr) can sometimes be best managed and understood using process flow diagrams and charts, as illustrated in. Web the concepts, implementation, and timing of major design transfer associated tasks (design verification, process validation, design validation, design outputs, and dmr) can sometimes be best managed and understood using process flow diagrams and charts, as illustrated in figure 1. What do the fda and iso 13485: Web design transfer begins. Web design transfer is a critical phase in the. Web what is design transfer? For companies navigating the stringent. It involves transferring a meticulously designed device from the research and development (r&d) environment to production and commercialization. Web design transfer is a pivotal phase that bridges the gap between concept and reality in medical device development. Web moving from medical device design to production can be a challenging process. Web design transfer is a critical phase in the development of medical devices, marking the transition from the design stage to manufacturing. Web transferring design to production is a process that can be fraught with risk for medical device developers. Take these points into consideration to enable a smooth design transfer. 5/5 (29k reviews) Web the design transfer process includes. Web what is design transfer? It involves transferring a meticulously designed device from the research and development (r&d) environment to production and commercialization. Web moving from medical device design to production can be a challenging process. 5/5 (29k reviews) Web the design transfer process includes a number of activities—like demonstrating successful design verification and validation and ensuring your device master. Web design transfer is a pivotal phase that bridges the gap between concept and reality in medical device development. Web transferring design to production is a process that can be fraught with risk for medical device developers. Web moving from medical device design to production can be a challenging process. Design transfer is the culmination of the medical device design. Effective design transfer requires a thorough assessment of the product's design documentation, selection of components, and careful definition of production methods. Web of these transitions, design transfer, the introduction of a design to production, may be the most important. And because of this, the process of transferring a medical device from product development to production (often referred to as “design. What do the fda and iso 13485: 2016 say about design transfer? Web design transfer is a critical phase in the development of medical devices, marking the transition from the design stage to manufacturing. Web design transfer begins. Web design transfer is a critical component outlined under the design control section of 21 cfr 820.30, which mandates that manufacturers establish. Design transfer is a component of the fda’s medical device quality system regulation design controls. Web design transfer is a critical component outlined under the design control section of 21 cfr 820.30, which mandates that manufacturers establish procedures ensuring accurate translation of device design into production specifications. Web the design transfer process includes a number of activities—like demonstrating successful design verification and validation and ensuring your device master record (dmr) is complete and accurate—that must occur before manufacturing can begin. Web design transfer is a pivotal phase that bridges the gap between concept and reality in medical device development. 5/5 (29k reviews) For companies navigating the stringent. Web what is design transfer? Web design transfer is a critical phase in the development of medical devices, marking the transition from the design stage to manufacturing. Web transferring design to production is a process that can be fraught with risk for medical device developers. Web moving from medical device design to production can be a challenging process. How can you be sure no one in your supply chain drops the ball as they assume control of the product you’ve taken so long to design and develop? And because of this, the process of transferring a medical device from product development to production (often referred to as “design transfer”) begins during design validation. It involves transferring a meticulously designed device from the research and development (r&d) environment to production and commercialization. Web design transfer begins. It ensures that design specifications are accurately and thoroughly translated into production specifications, leading to consistent, safe, and effective devices. Take these points into consideration to enable a smooth design transfer.
6 Ways to Transfer your Embroidery Design to Fabric Create Whimsy

Easiest Design Transfer Method You'll Ever Find Makely Simple

Design Transfer A practical Approach QualityMedDev

Embroidery design transfer techniques and tools, How to transfer a pattern

Easy Design Transfer with Graphite • Craving Some Creativity
Best Practices for Design Transfer Jama Software

Try this Embroidery Design Transfer Tip!
.jpg)
Design Transfer Methods Arts & Crafts Ideas
ELLA'S CRAFT CREATIONS Design Transfer tutorial......My way ! And my
Design Transfer A practical Approach QualityMedDev
2016 Say About Design Transfer?
Web Of These Transitions, Design Transfer, The Introduction Of A Design To Production, May Be The Most Important.
Effective Design Transfer Requires A Thorough Assessment Of The Product's Design Documentation, Selection Of Components, And Careful Definition Of Production Methods.
What Do The Fda And Iso 13485:
Related Post: