Design History File Medical Device
Design History File Medical Device - Web a design history file (dhf) shows the design history of a medical device. Web as part of these regulatory requirements, manufacturers must establish and maintain a medical device design history file (dhf) for each type of device. This guide breaks down everything you. The food and drug administration (fda or the agency), the us regulating authority in the sphere of medical devices, has published a guidance document. It includes or references records generated to. Web the design history file (dhf): Web a design history file (dhf) is a comprehensive documentation containing all the records and information related to the design and development of a product. The final step in the fda’s design controls process, as required by 21. Web the design history file, or dhf, is part of regulation introduced in 1990 when the u.s. Web the fda requires a design history file dhf in 21 cfr part 820 (these are the “quality system regulations”). Web the design history file, or dhf, is part of regulation introduced in 1990 when the u.s. Web learn what a design history file (dhf) is, why it is important for medical device development, and what it should include. It is used to provide evidence that all the design control procedures were appropriately. Your design history file (dhf) is an. Web one of the common causes of angst among medical device developers is preparing their design history file (dhf) so that it is up to scratch for the scrutiny of the fda. Is a leading authority in. Web learn about the importance, history, and requirements of design controls for medical devices from the u.s. Web the requirements for a design. Web the design history file, or dhf, is part of regulation introduced in 1990 when the u.s. It includes or references records generated to. Its purpose is to demonstrate that the device. The food and drug administration (fda or the agency), the us regulating authority in the sphere of medical devices, has published a guidance document. Your design history file. Web the design history file serves as a comprehensive and structured record of the entire design and development process of a medical device. “each manufacturer shall establish and maintain a dhf for each type of device. The final step in the fda’s design controls process, as required by 21. Web a medical device design history file (dhf) is a critical. Is a leading authority in. Web learn about the importance, history, and requirements of design controls for medical devices from the u.s. Web a design history file (dhf) is a comprehensive documentation containing all the records and information related to the design and development of a product. The dhf is a compilation of records which describes the design history of. Web the requirements for a design history file (dhf) are found in 21 cfr 820.30j: Is a leading authority in. Web facing an iso 13485 or fda 21 cfr 820 audit? Web according to fda 21 cfr 820.30 (j), a design history file (dhf) is a compilation of records that carries the design history of a finished medical device. Web. Is a collection of documents that describe the design and development activities of a medical device. “each manufacturer shall establish and maintain a dhf for each type of device. The food and drug administration (fda or the agency), the us regulating authority in the sphere of medical devices, has published a guidance document. Web for medical device developers assembling and. Web download our design history file (dhf) pdf guide to: The primary purposes of a dhf are:. Web the fda requires a design history file dhf in 21 cfr part 820 (these are the “quality system regulations”). This guide breaks down everything you. Web the design history file, or dhf, is part of regulation introduced in 1990 when the u.s. Web a design history file (dhf) is used throughout the design and development process of a medical device. Web learn how to create and manage a design history file (dhf) or technical documentation (td) for medical devices, complying with fda and mdr regulations. Find out the difference between dhf,. Work with trusted expertstherapeutic expertiseinnovative & full service Is a collection. The food and drug administration (fda or the agency), the us regulating authority in the sphere of medical devices, has published a guidance document. Web a design history file (dhf) is used throughout the design and development process of a medical device. Congress passed the safe medical devices act, which established new standards for. Web learn how to create and. A design history file (dhf) and how do they relate to each other? The food and drug administration (fda or the agency), the us regulating authority in the sphere of medical devices, has published a guidance document. Dhf should not be confused with the device history. Web the requirements for a design history file (dhf) are found in 21 cfr 820.30j: Web the design history file serves as a comprehensive and structured record of the entire design and development process of a medical device. “each manufacturer shall establish and maintain a dhf for each type of device. Web download our design history file (dhf) pdf guide to: Web learn what a design history file (dhf) is, why it is important for medical device development, and what it should include. Web the design history file (dhf): Web the design history file, or dhf, is part of regulation introduced in 1990 when the u.s. Web as part of these regulatory requirements, manufacturers must establish and maintain a medical device design history file (dhf) for each type of device. Your design history file (dhf) is an invaluable piece of the puzzle. It is used to provide evidence that all the design control procedures were appropriately. Is a leading authority in. Web according to the fda 21 cfr 820.30 design history file is a set of consolidated documents for evaluating the processes involved in the. The dhf is a compilation of records which describes the design history of a finished device.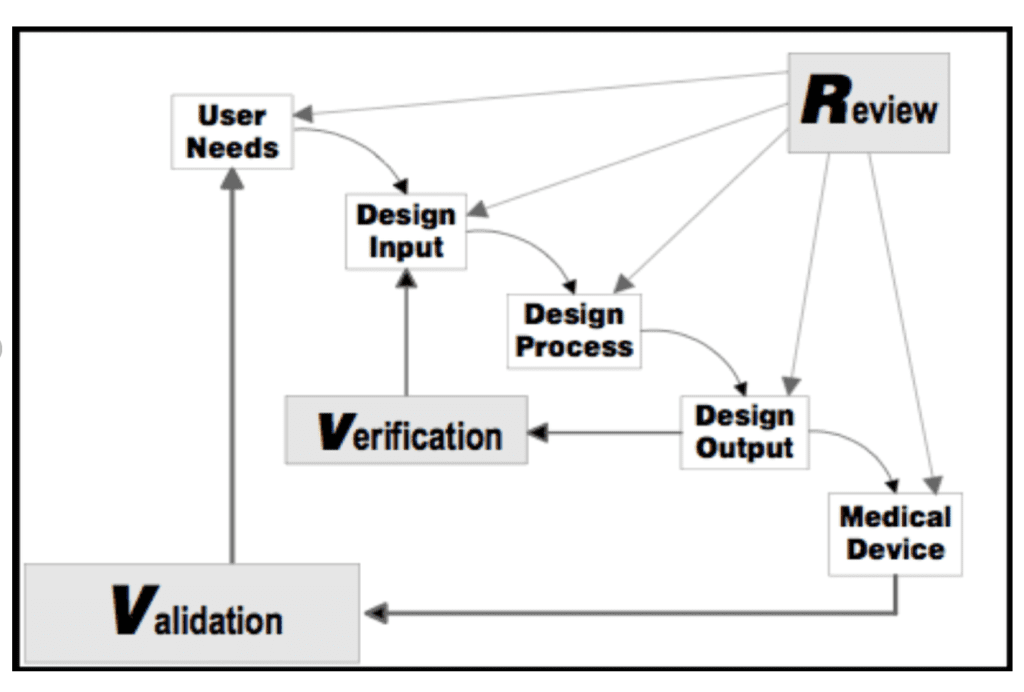
Medical Device Design History File Template

Design History File (DHF) SOP QMDocs Quality Management System Templates
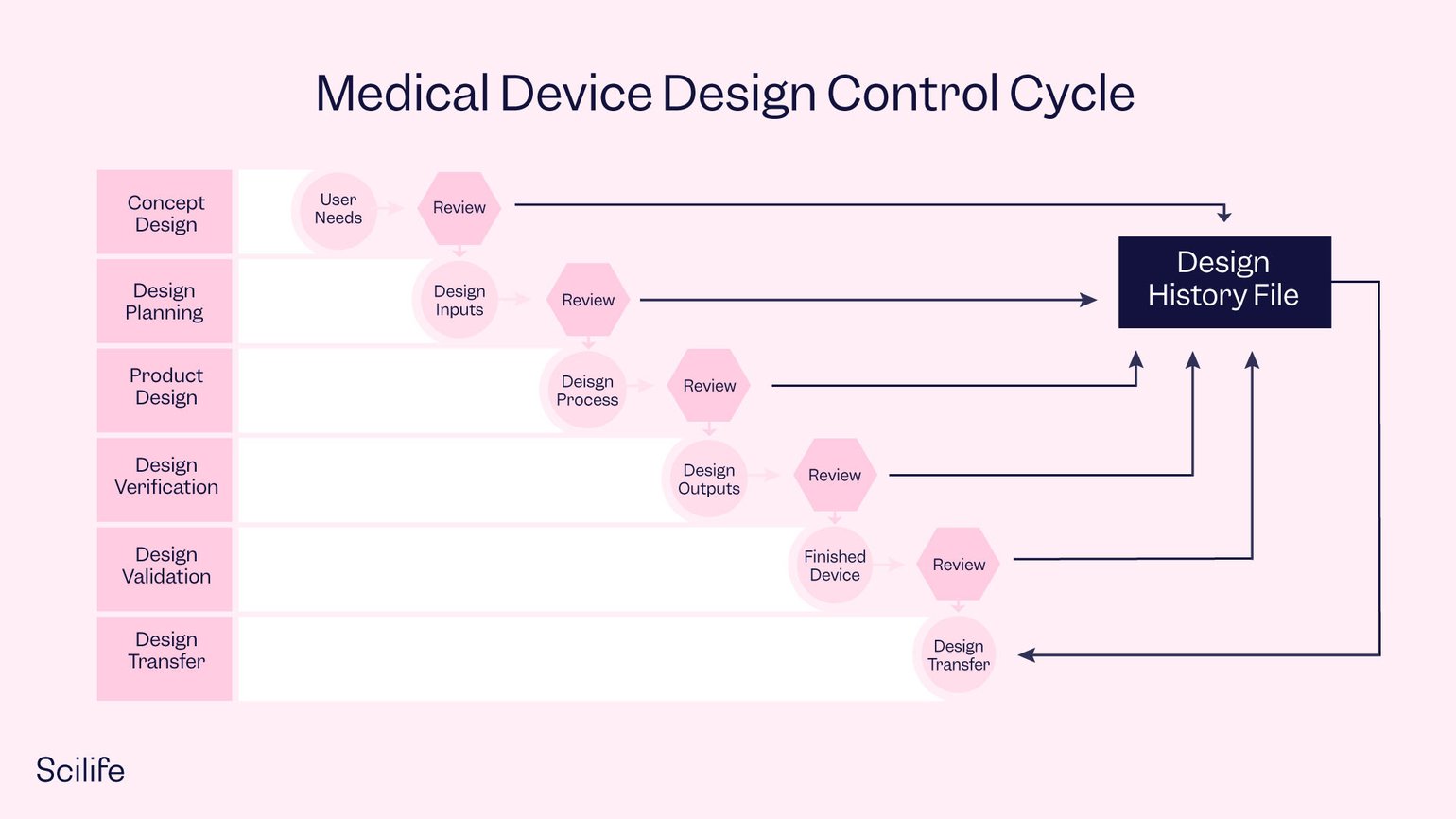
What is Design History File (DHF)? Complete definition Scilife
What is Design History File? Why it is Important for Medical Device
Medical Device Design History File Template

Assembling a Design History File (DHF) for your medical device

Design History Files Everything You Should Know

DHF Template Format and Content of Design History File Medical Device
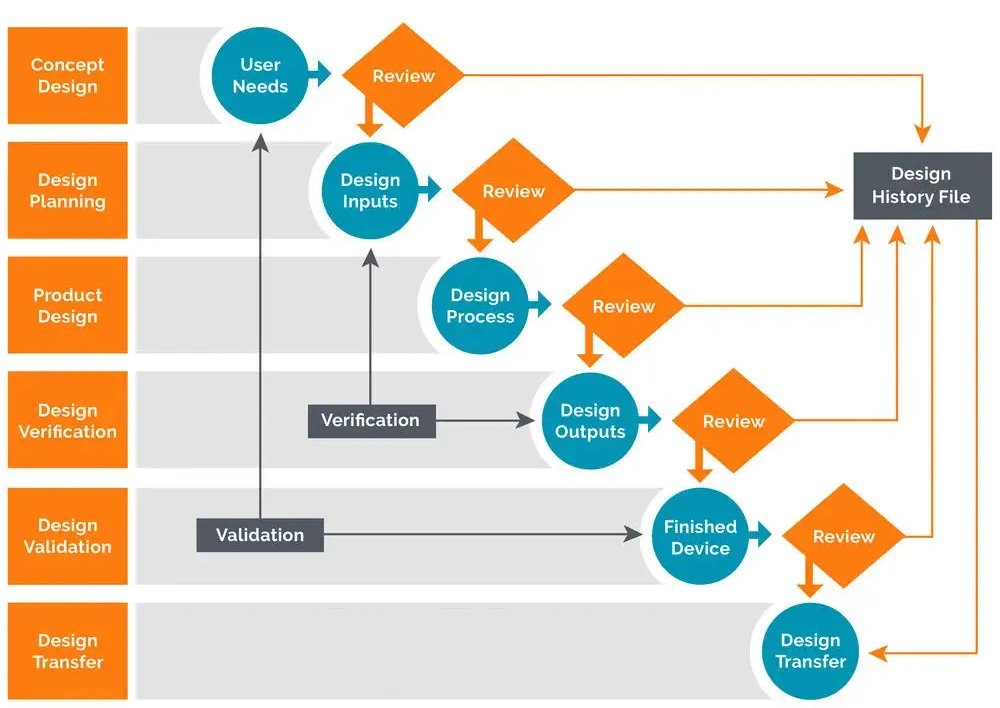
What is Design History File? Why it is Important for Medical Device

The Medical Design History File — Garrett Technologies, Inc.
Web A Medical Device Design History File (Dhf) Is A Critical Document Used In Medical Devices’ Development And Regulatory Compliance Process.
A Good Dhf Is A Logical, Structured And Ordered Compilation Of Your Medical Device Design Control Activities.
Web What Is A Design History File (Dhf)?
Web A Design History File (Dhf) Is A Comprehensive Documentation Containing All The Records And Information Related To The Design And Development Of A Product.
Related Post: