Design Controls For The Medical Device Industry
Design Controls For The Medical Device Industry - With this guide, i plan to share valuable insights to explain what design controls are, how to address them, and how they benefit your medical device product development efforts. By now you know that for market entry your medical device needs to qualify certain regulatory requirements and standards. Web for questions about design control documentation, see the guidance for industry design control guidance for. The path of a medical device from idea to final application can seem quite long. Web this third edition provides a substantial comprehensive review of the latest design control requirements, as well as proven tools and techniques to ensure a company's design control program evolves in accordance with current industry practice. Explores proven techniques and methods for compliance. The following examples highlight 5 key design applications when implementing sensory design in medical devices. Let's explore the key elements of iso 13485 that will help you manage the process effectively. Web principles of sensory design. Web visit panasonic's automation controls download center for comprehensive access to cad data, manuals, software, and technical guides for our electronic components and industrial devices. Explore relays, connectors, switches, encoders, potentiometers, touch solutions, and more for your needs. Web this documentation process is widely known as “design controls” and its purpose is to ensure that medical devices meet user needs, intended uses, and specified requirements. Why your design outputs need to be more than a drawing and their relationship to your dmr. Web safe medical. Web this third edition provides a substantial comprehensive review of the latest design control requirements, as well as proven tools and techniques to ensure a company's design control program evolves in accordance with current industry practice. Web design controls play a crucial role in the medical device development process by providing a systematic approach to manage risks, design requirements, and. The global harmonization task force. Web for questions about design control documentation, see the guidance for industry design control guidance for. Web safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements for medical devices. Jedec standards encompass virtually every key standard for semiconductor memory in the. Contributes fresh templates. Explores proven techniques and methods for compliance. Why your design outputs need to be more than a drawing and their relationship to your dmr. With this guide, i plan to share valuable insights to explain what design controls are, how to address them, and how they benefit your medical device product development efforts. The global harmonization task force. Web even. Sensory features by user group. A combination of sensory designs aids how efficiently people. Web you'll learn about design outputs, device master record (dmr), design verification and validation (v&v), design transfer and regulatory submissions. Elevate your understanding and mastery of design control for a seamless journey in medical device development. Web visit panasonic's automation controls download center for comprehensive access. Web for questions about design control documentation, see the guidance for industry design control guidance for. Web you'll learn about design outputs, device master record (dmr), design verification and validation (v&v), design transfer and regulatory submissions. From concept to market, navigate every stage with confidence and precision. The following examples highlight 5 key design applications when implementing sensory design in. Semiconductor memory plays an essential role in the development and functions of countless electronic devices ranging from computers and servers to gaming consoles to televisions and telecommunications products. Elevate your understanding and mastery of design control for a seamless journey in medical device development. Iec & iso regulations and compliance. Web the book includes a review of the design control. With this guide, i plan to share valuable insights to explain what design controls are, how to address them, and how they benefit your medical device product development efforts. Let's explore the key elements of iso 13485 that will help you manage the process effectively. Web even the best and brightest in the medical device industry can struggle to build. Web principles of sensory design. By now you know that for market entry your medical device needs to qualify certain regulatory requirements and standards. Web even the best and brightest in the medical device industry can struggle to build a design control process that ensures safety and efficacy, and enables high quality standards to be met throughout the product life. A combination of sensory designs aids how efficiently people. Web discover how design control ensures quality in medical devices. The global harmonization task force. Semiconductor memory plays an essential role in the development and functions of countless electronic devices ranging from computers and servers to gaming consoles to televisions and telecommunications products. Web even the best and brightest in the. Web visit panasonic's automation controls download center for comprehensive access to cad data, manuals, software, and technical guides for our electronic components and industrial devices. Web design control guidance for medical device manufacturers. •they set medical device quality systems apart Web principles of sensory design. The following examples highlight 5 key design applications when implementing sensory design in medical devices. Learn about fda guidance, documentation, and the design control process flow chart. Sensory features by user group. Web this documentation process is widely known as “design controls” and its purpose is to ensure that medical devices meet user needs, intended uses, and specified requirements. By now you know that for market entry your medical device needs to qualify certain regulatory requirements and standards. Iec & iso regulations and compliance. The global harmonization task force. Jedec standards encompass virtually every key standard for semiconductor memory in the. However, first and foremost, the most important feature is that medical devices are designed and developed safely. A combination of sensory designs aids how efficiently people. Web in this article, we will focus more on medical device design, design controls, and compliance. Look through the blue transparent top case and side caps to see metallic, silver interior parts shine through from inside.
Design controls for the medical device industry, Computers & Tech
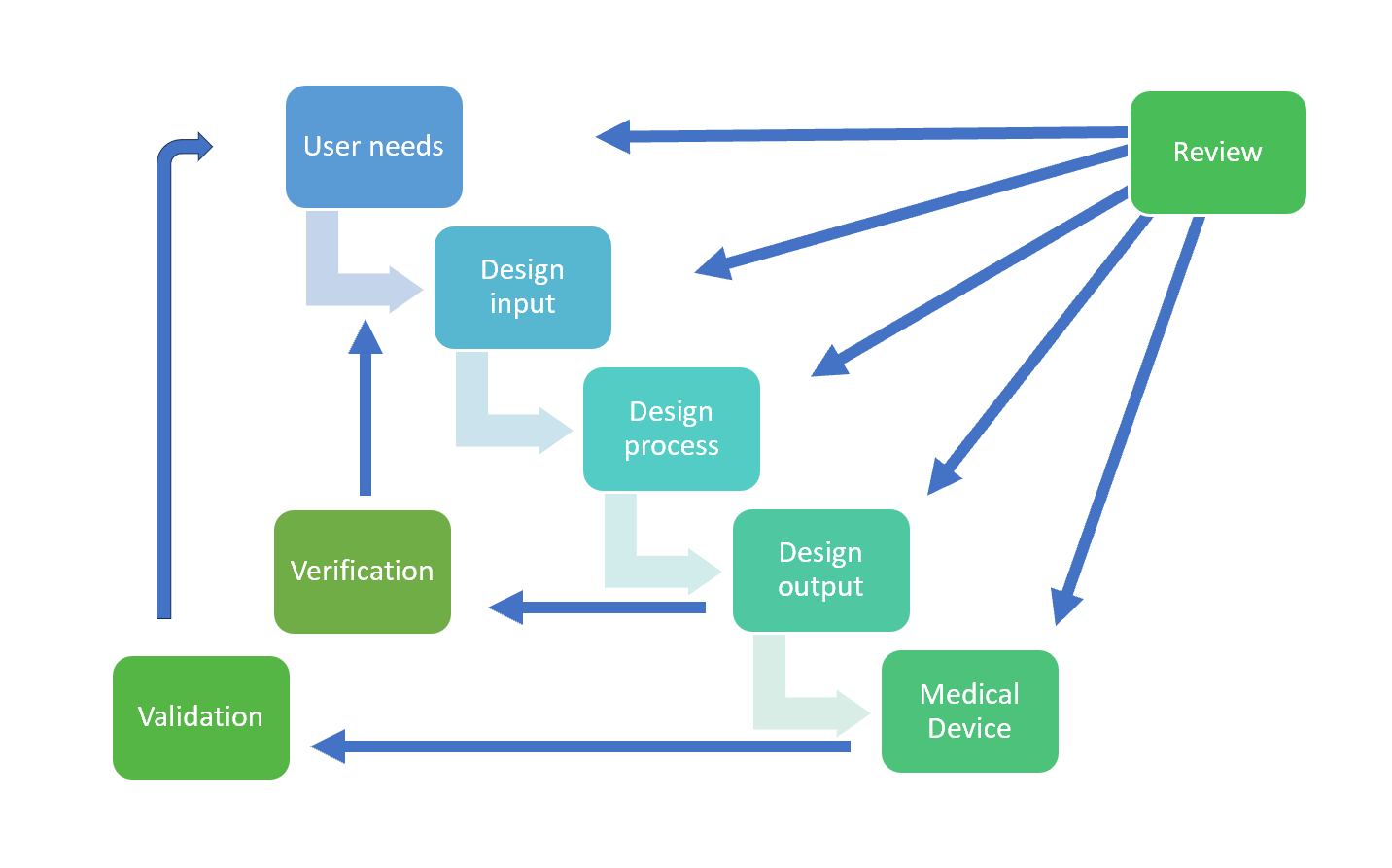
Design controls in medical device development Ensuring safety and
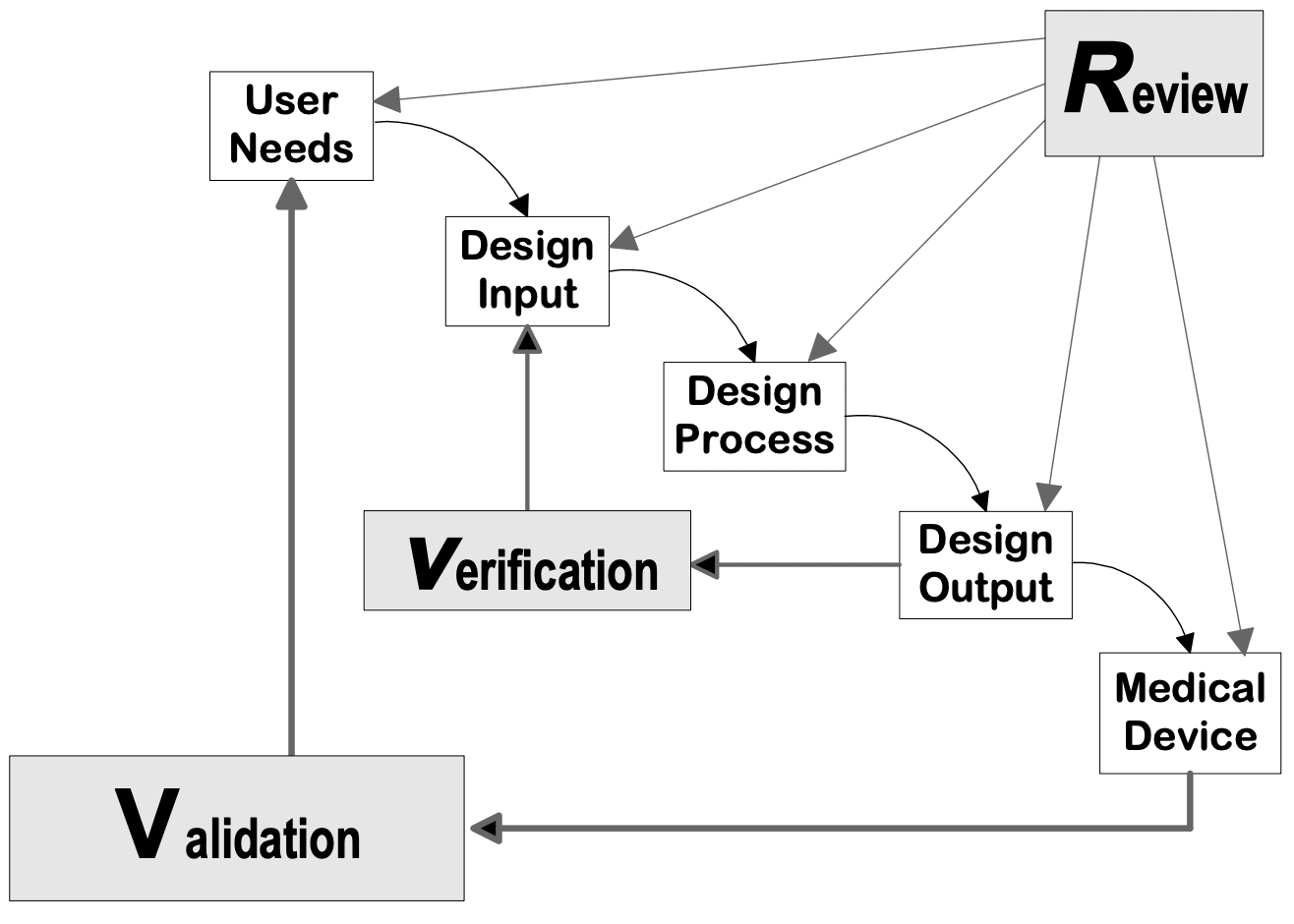
A guide to FDA Design Controls for your medical device
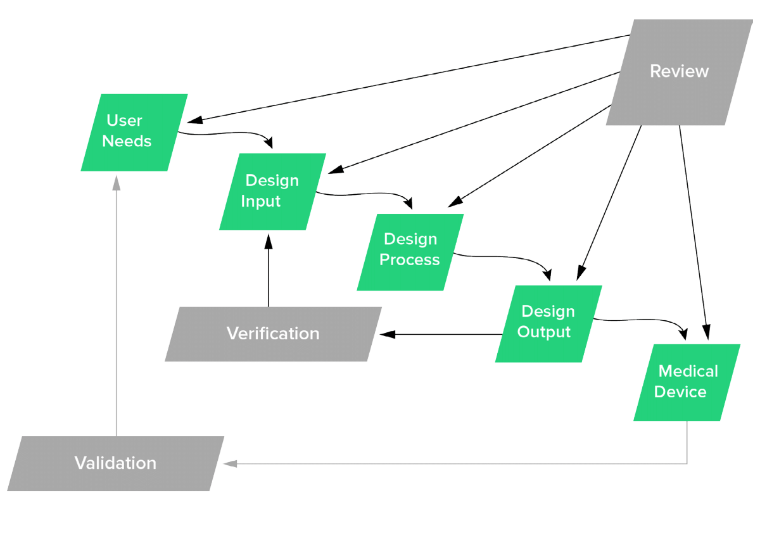
The Ultimate Guide To Design Controls For Medical Device Companies
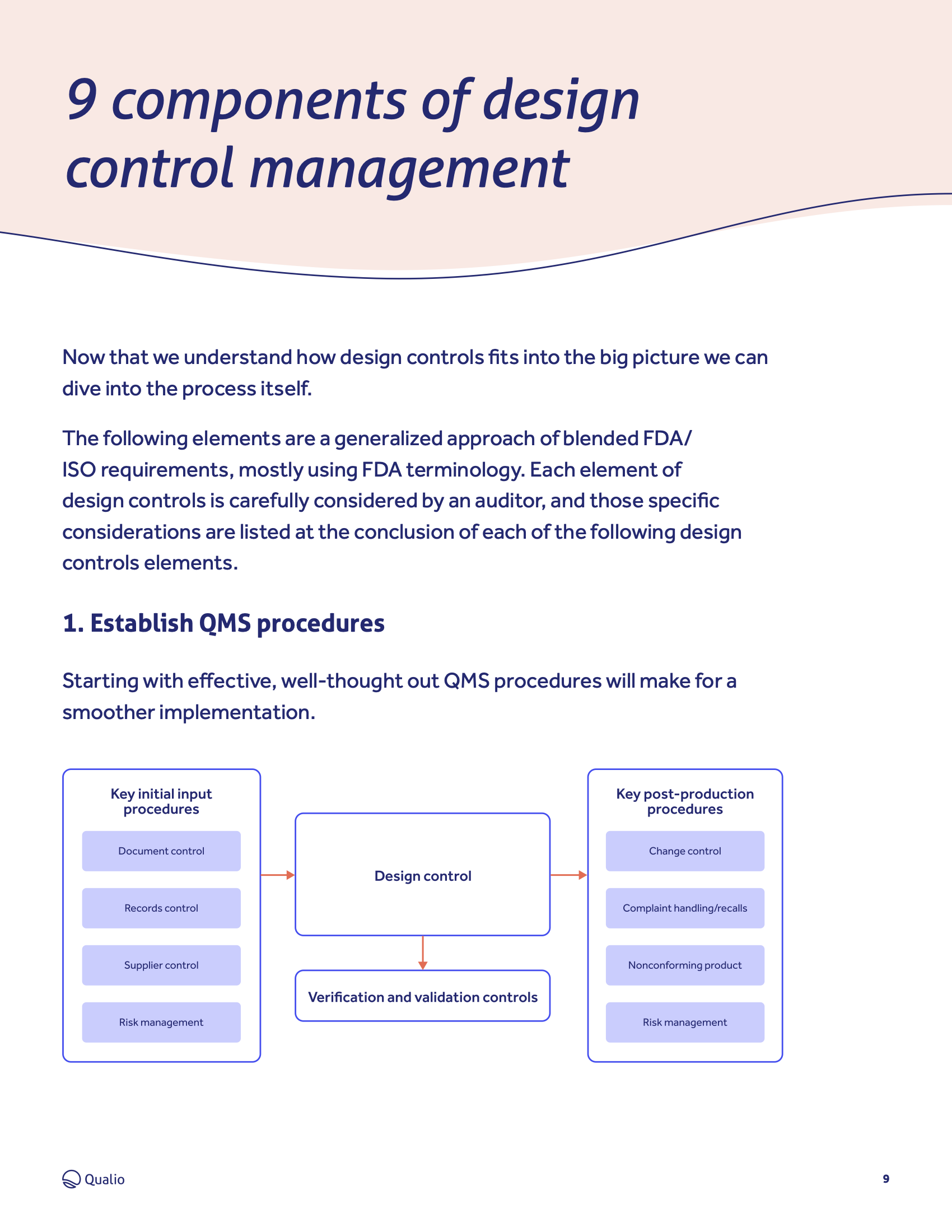
Ultimate guide to medical device design controls
Design Controls For The Medical Device Industry by MARIE PDF Risk

Basics of Medical Device Design Controls What, Why, and How Oriel

Ultimate guide to medical device design controls
Design Controls For The Medical Device Industry, Third Edition Marie

Design Controls for the Medical Device Industry
Web The Purpose Of Design Control For Medical Devices Is To Keep Patient And User Risk Low Without Compromising The Integrity Of The Product Manufactured.
Web Safe Medical Device Act Of 1990 Authorized Fda To Add Design Controls To The Current Good Manufacturing Practice (Cgmp) Requirements For Medical Devices.
From Concept To Market, Navigate Every Stage With Confidence And Precision.
Explore Relays, Connectors, Switches, Encoders, Potentiometers, Touch Solutions, And More For Your Needs.
Related Post:

