Design A Protocol
Design A Protocol - A written protocol facilitates high. Web using this protocol model, we created protocol designer to be a web application where users can: Web a communication protocol is a system of rules that allows two or more entities of a communications system to transmit information via any variation of a physical quantity. All the details of the main investigator must be reported in the first paragraph. Architectures, mechanisms, principles, issues, and pitfalls for protocol design from a conceptual viewpoint (examples!) (taking. String together a series of actions to produce a protocol. A written protocol facilitates high. A growing number of journals, granting. This will allow each participant to know who ask for in case of doubts or criticalities during the research. Web a research protocol must start from the definition of the coordinator of the whole study: String together a series of actions to produce a protocol. Web a good protocol should delineate the research questions and outline the research process, show how the design will help achieve the objectives, demonstrate how the study will be. Web your protocol should should specify: This will allow each participant to know who ask for in case of doubts or. Which programs use the protocol? Web guidelines for designing a clinical study protocol. Write a server that understands that protocol; Correct design and implementation prompts the use of formal languages that su. Web the first step in writing a protocol is to decide on the appropriate study design to address the research question. Clinical research is conducted according to a plan (a protocol) or an action plan. To draft a sound scientific design of a clinical research study, the medical writer at the tgh, office of. Web this approach is aimed to assess feasibility of a study to be conducted at particular institution (i.e., assess availability of clinical services, equipment needed to. String. The quality of science is often improved when study objectives and methods are clearly thought through and described. Important topics for application protocol. Web using this protocol model, we created protocol designer to be a web application where users can: Which programs use the protocol? The protocol demonstrates the guidelines for conducting the. Web the research protocol is a document that describes the background, rationale, objective (s), design, methodology, statistical considerations and organization. A written protocol facilitates high. Web a good protocol should delineate the research questions and outline the research process, show how the design will help achieve the objectives, demonstrate how the study will be. Web your protocol should should specify:. Clinical research is conducted according to a plan (a protocol) or an action plan. Specify instruments, labware, and liquids. Ls, that should be suitable for industrial application. A growing number of journals, granting. To draft a sound scientific design of a clinical research study, the medical writer at the tgh, office of. A growing number of journals, granting. Web the first step in writing a protocol is to decide on the appropriate study design to address the research question. Important topics for application protocol. Web a communication protocol is a system of rules that allows two or more entities of a communications system to transmit information via any variation of a physical. Web write a browser or user agent of some kinds that understands that protocol, both in its url form and in the actual data format; Web creating a protocol is an important step in ensuring that your research is rigorous, ethical, feasible, and reproducible. Ls, that should be suitable for industrial application. All the details of the main investigator must. Configure actions (like heat, transfer, mix, etc.) using forms with customizable inputs. Web writing a protocol can be a daunting task, but with the right approach, it can be an efficient and effective process. Web what is a protocol? A growing number of journals, granting. Web these principles provide a vocabulary for protocol and system designers, administrators and many other. Preferably, have a specification for the protocol so that browser and server can continue to work together. The quality of science is often improved when study objectives and methods are clearly thought through and described. A written protocol facilitates high. Important topics for application protocol. To draft a sound scientific design of a clinical research study, the medical writer at. A written protocol facilitates high. Web the research protocol is a document that describes the background, rationale, objective (s), design, methodology, statistical considerations and organization. Ls, that should be suitable for industrial application. Web guidelines for designing a clinical study protocol. String together a series of actions to produce a protocol. To draft a sound scientific design of a clinical research study, the medical writer at the tgh, office of. Web the first step in writing a protocol is to decide on the appropriate study design to address the research question. Web this not only enables us to describe protocols in a concise manner, but also to reason about many of their interesting properties and formally to prove certain aspects of their. The network protocol specifies how programs send data across the network. Architectures, mechanisms, principles, issues, and pitfalls for protocol design from a conceptual viewpoint (examples!) (taking. The quality of science is often improved when study objectives and methods are clearly thought through and described. All the details of the main investigator must be reported in the first paragraph. Web your protocol should should specify: Specify instruments, labware, and liquids. Web a good protocol should delineate the research questions and outline the research process, show how the design will help achieve the objectives, demonstrate how the study will be. Web these principles provide a vocabulary for protocol and system designers, administrators and many other stakeholders to evaluate protocols, to get involved in ongoing.
Multiprotocol design several protocols It talks HTTP and SOAP with
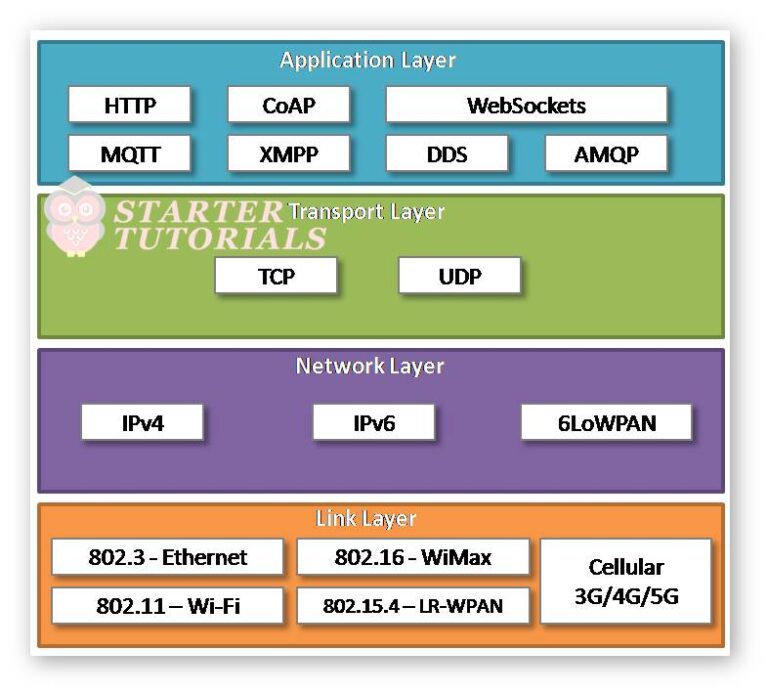
Physical Design of IoT IoT Tutorial for Beginners
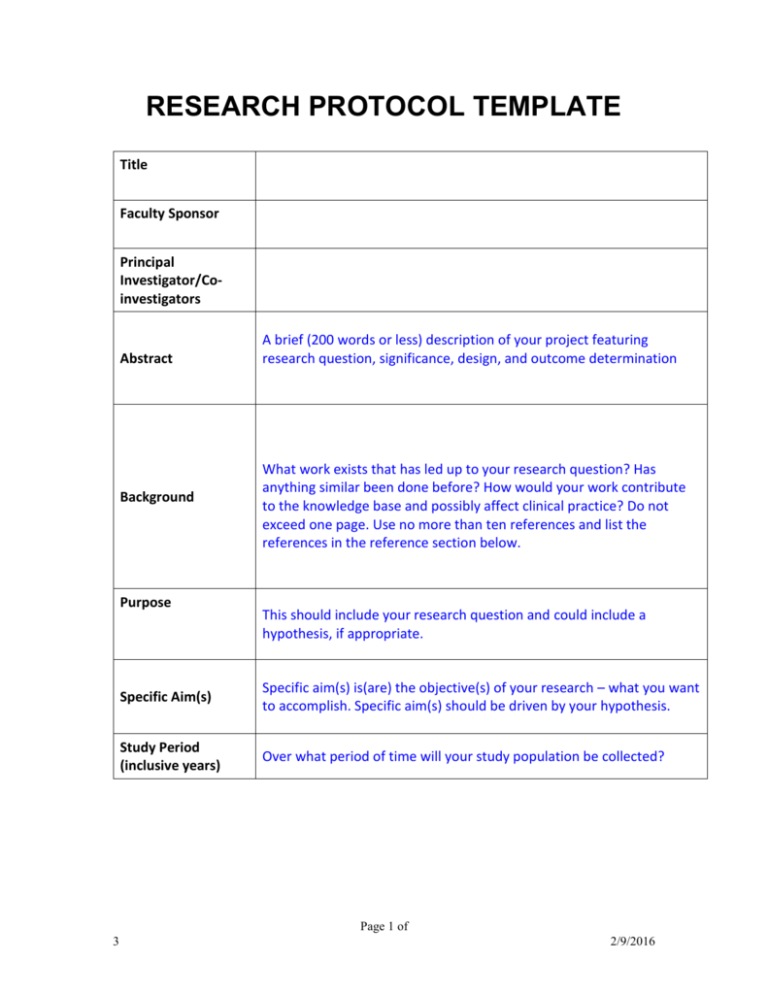
research protocol template

PPT Protocol Design PowerPoint Presentation, free download ID1614900

guidelines for writing a research protocol
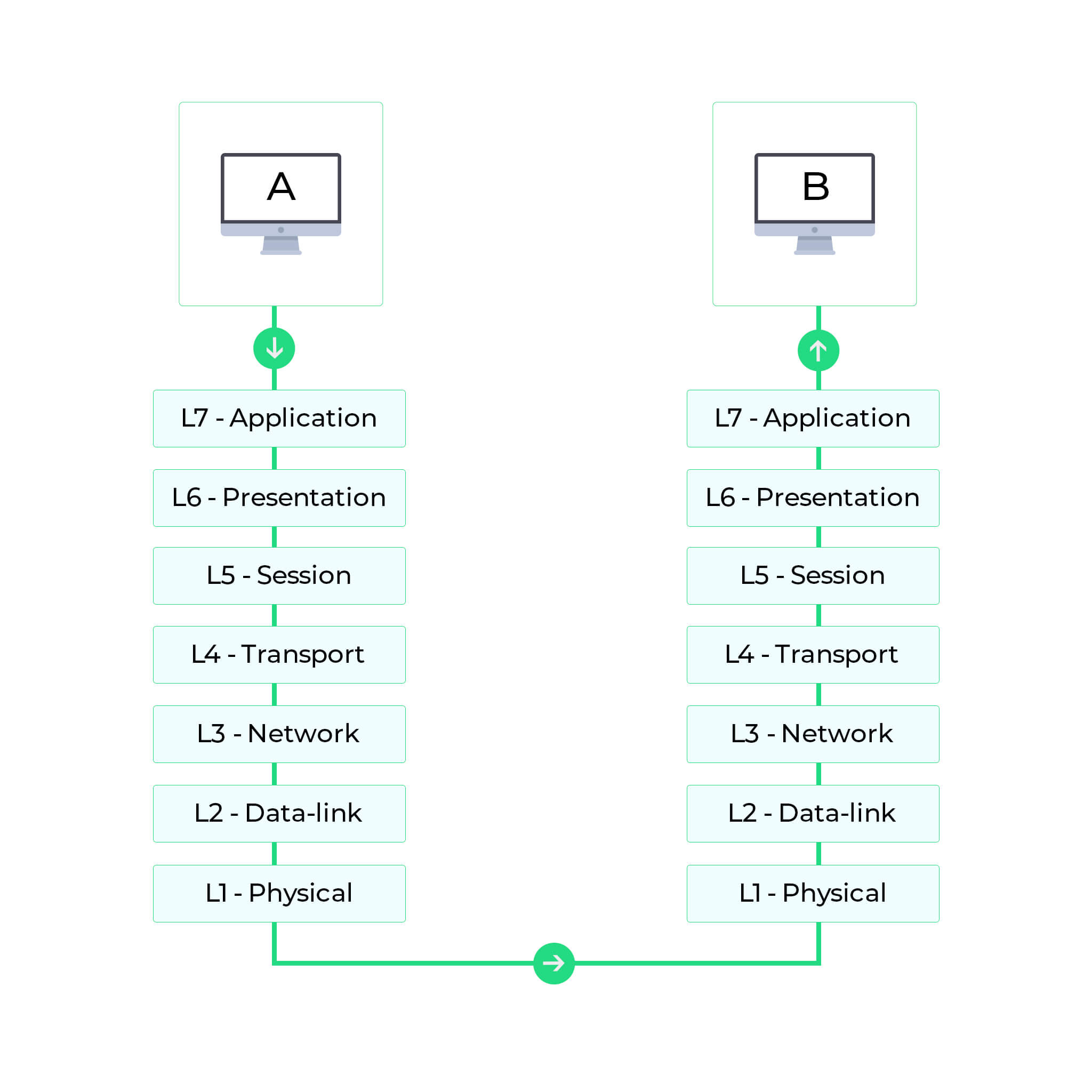
Types of Network Protocols The Ultimate Guide Blink Protocol

PPT Network Protocol Software Design and Implementation PowerPoint

PPT Protocol Design PowerPoint Presentation, free download ID1614900

How to Design a Clinical Trial Protocol • Dicentra

PPT Protocol Design PowerPoint Presentation, free download ID1614900
Web Write A Browser Or User Agent Of Some Kinds That Understands That Protocol, Both In Its Url Form And In The Actual Data Format;
Web Creating A Protocol Is An Important Step In Ensuring That Your Research Is Rigorous, Ethical, Feasible, And Reproducible.
Configure Actions (Like Heat, Transfer, Mix, Etc.) Using Forms With Customizable Inputs.
This Will Allow Each Participant To Know Who Ask For In Case Of Doubts Or Criticalities During The Research.
Related Post: