Dea Controlled Substance Log Template
Dea Controlled Substance Log Template - In our opinion, the use of bound controlled substance logbooks such as the third edition of the aaha controlled substance logs meet the minimum requirements for use in all 50 us states and canada. Web prescribing, administering, and dispensing controlled substances under the controlled substances act (csa), 21 u.s.c. Web the updated aaha controlled substance logs ensure veterinary professionals meet dea recordkeeping requirements across all 50 states and canada. To prevent diversion and misuse of these substances while ensuring an adequate and uninterrupted supply is available to meet the country’s legitimate medical, scientific, and research needs. Modify dea registration to stop being a collector; Web controlled substances inventory (word) controlled substances receipt log (word) controlled substances use log (word) up next: E.g., syringe hub loss or amount remaining due to expiring. For questions, contact eh&s at [email protected]. With respect to pharmaceutical controlled substances, dea’s responsibility is twofold: Reviewed by dea experts, these logs help achieve compliance, safeguard against errors, and provide internal accountability. Reviewed by dea experts, these logs help achieve compliance, safeguard against errors, and provide internal accountability. Modify dea registration to stop being a collector; (1) material drawn up for dosing that was not used or could not be fully extracted; Modify existing collector registration information Web the updated aaha controlled substance logs ensure veterinary professionals meet dea recordkeeping requirements across. Reviewed by dea experts, these logs help achieve compliance, safeguard against errors, and provide internal accountability. Web the dea requires you to keep accurate records and account for all of your controlled substances. Web the updated aaha controlled substance logs ensure veterinary professionals meet dea recordkeeping requirements across all 50 states and canada. Web modify eligible dea registration to collect. Web the updated aaha controlled substance logs ensure veterinary professionals meet dea recordkeeping requirements across all 50 states and canada. Modify existing collector registration information Web prescribing, administering, and dispensing controlled substances under the controlled substances act (csa), 21 u.s.c. In our opinion, the use of bound controlled substance logbooks such as the third edition of the aaha controlled substance. Web the updated aaha controlled substance logs ensure veterinary professionals meet dea recordkeeping requirements across all 50 states and canada. For questions, contact eh&s at [email protected]. With respect to pharmaceutical controlled substances, dea’s responsibility is twofold: Modify dea registration to stop being a collector; Modify existing collector registration information Modify existing collector registration information E.g., syringe hub loss or amount remaining due to expiring. Web modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); In our opinion, the use of bound controlled substance logbooks such as the third edition of the aaha controlled substance logs meet the minimum requirements for use in all 50. Web prescribing, administering, and dispensing controlled substances under the controlled substances act (csa), 21 u.s.c. Web the updated aaha controlled substance logs ensure veterinary professionals meet dea recordkeeping requirements across all 50 states and canada. In our opinion, the use of bound controlled substance logbooks such as the third edition of the aaha controlled substance logs meet the minimum requirements. Reviewed by dea experts, these logs help achieve compliance, safeguard against errors, and provide internal accountability. Web prescribing, administering, and dispensing controlled substances under the controlled substances act (csa), 21 u.s.c. (1) material drawn up for dosing that was not used or could not be fully extracted; With respect to pharmaceutical controlled substances, dea’s responsibility is twofold: For questions, contact. Web dea controlled substances usage log. For questions, contact eh&s at [email protected]. In our opinion, the use of bound controlled substance logbooks such as the third edition of the aaha controlled substance logs meet the minimum requirements for use in all 50 us states and canada. Web modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g.,. In our opinion, the use of bound controlled substance logbooks such as the third edition of the aaha controlled substance logs meet the minimum requirements for use in all 50 us states and canada. Modify dea registration to stop being a collector; Web the dea requires you to keep accurate records and account for all of your controlled substances. With. Modify dea registration to stop being a collector; E.g., syringe hub loss or amount remaining due to expiring. To prevent diversion and misuse of these substances while ensuring an adequate and uninterrupted supply is available to meet the country’s legitimate medical, scientific, and research needs. Web modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients);. In our opinion, the use of bound controlled substance logbooks such as the third edition of the aaha controlled substance logs meet the minimum requirements for use in all 50 us states and canada. Modify existing collector registration information Modify dea registration to stop being a collector; Web prescribing, administering, and dispensing controlled substances under the controlled substances act (csa), 21 u.s.c. Web dea controlled substances usage log. Web the dea requires you to keep accurate records and account for all of your controlled substances. Web the updated aaha controlled substance logs ensure veterinary professionals meet dea recordkeeping requirements across all 50 states and canada. Reviewed by dea experts, these logs help achieve compliance, safeguard against errors, and provide internal accountability. With respect to pharmaceutical controlled substances, dea’s responsibility is twofold: Web modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); For questions, contact eh&s at [email protected]. To prevent diversion and misuse of these substances while ensuring an adequate and uninterrupted supply is available to meet the country’s legitimate medical, scientific, and research needs.
Controlled Substance Usage Log printable pdf download
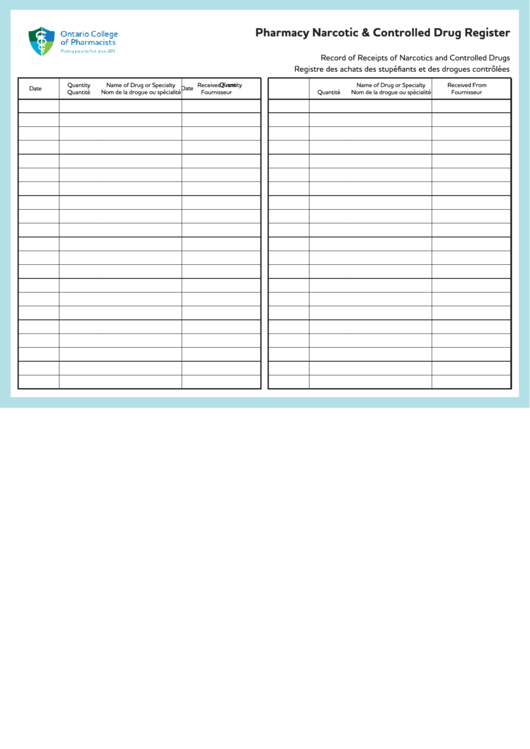
Free Printable Controlled Substance Log

Free Printable Controlled Substance Log
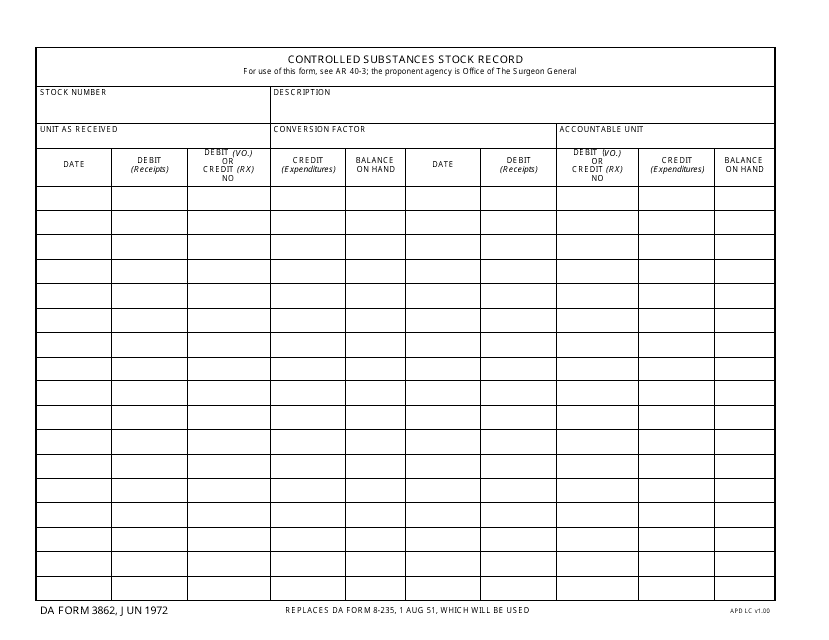
DA Form 3862 Download Fillable PDF, Controlled Substances Stock Record
Printable Controlled Substance Inventory Log

Free Printable Controlled Substance Log
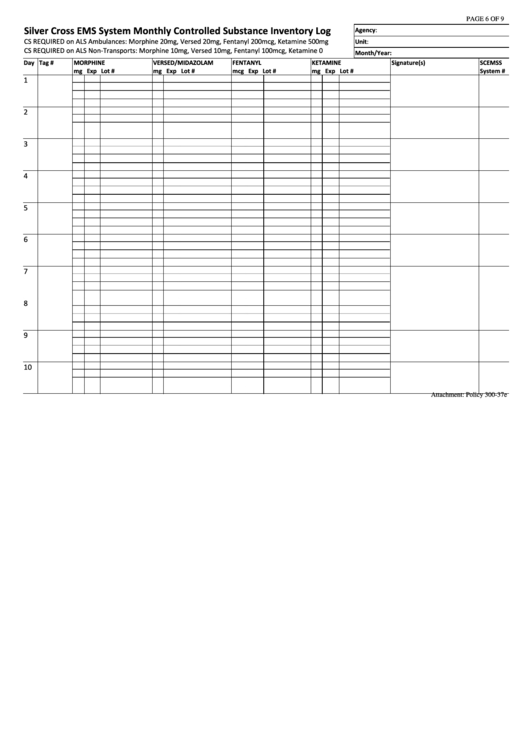
Free Printable Controlled Substance Log
Printable Controlled Substance Inventory Log

Free Printable Controlled Substance Log Customize and Print

Free Printable Controlled Substance Log Printable Word Searches
Web Controlled Substances Inventory (Word) Controlled Substances Receipt Log (Word) Controlled Substances Use Log (Word) Up Next:
E.g., Syringe Hub Loss Or Amount Remaining Due To Expiring.
(1) Material Drawn Up For Dosing That Was Not Used Or Could Not Be Fully Extracted;
Related Post:

