Consort Diagram Template
Consort Diagram Template - Analysed n = excluded from analysis n = give reasons n =. Web the consort 2010 statement is this paper including the 25 item checklist in the table (table 1) and the flow diagram (figure 1). Mary and tim last modified by: Revised template of the consort diagram showing the flow of participants through each stage of a randomized trial. It is aimed at primary reports of rcts with two group, parallel designs. Mary and tim created date: Web the expanded consort figure draws on theory, a prior meeting and recent recommendations for reporting factors related to external validity. It expands the basic consort flow diagram for clinical trials to summarize external validity and contextual factors more concisely and transparently. It provides guidance for reporting all randomised controlled trials, but focuses on the most common design type— individually randomised, two group, parallel trials. It provides guidance for reporting all randomised controlled trials, but focuses on the most common design type—individually randomised, two group, parallel trials. Mary and tim created date: Allocated to intervention n = received intervention n = did not receive intervention n = give reasons n =. It is aimed at primary reports of rcts with two group, parallel designs. Web the consort statement (or simply consort) comprises a checklist of essential items that should be included in reports of rcts and a. Allocated to intervention n = received intervention n = did not receive intervention n = give reasons n =. It provides guidance for reporting all randomised controlled trials, but focuses on the most common design type—individually randomised, two group, parallel trials. Web the expanded consort figure draws on theory, a prior meeting and recent recommendations for reporting factors related to. Mary and tim created date: Allocated to intervention n = received intervention n = did not receive intervention n = give reasons n =. Analysed n = excluded from analysis n = give reasons n =. Web consort 2010 flow diagram assessed for eligibility (n= ) excluded (n= ) The flow diagram can be accessed via the original published paper. = give reasons n =. Allocated to intervention n = received intervention n = did not receive intervention n = give reasons n =. It provides guidance for reporting all randomised controlled trials, but focuses on the most common design type— individually randomised, two group, parallel trials. Mary and tim last modified by: The flow diagram can be accessed via. Web the consort statement (or simply consort) comprises a checklist of essential items that should be included in reports of rcts and a diagram for documenting the flow of participants through a trial. Mary and tim created date: = give reasons n =. Web the consort 2010 statement is this paper including the 25 item checklist in the table (table. It is aimed at primary reports of rcts with two group, parallel designs. The flow diagram can be accessed via the original published paper by following the pubmed links in the full bibliographic reference section of this web page. Web consort 2010 flow diagram assessed for eligibility (n= ) excluded (n= ) = give reasons n =. Analysed n =. It provides guidance for reporting all randomised controlled trials, but focuses on the most common design type—individually randomised, two group, parallel trials. Web the consort 2010 statement is this paper including the 25 item checklist in the table (table 1) and the flow diagram (figure 1). It expands the basic consort flow diagram for clinical trials to summarize external validity. It provides guidance for reporting all randomised controlled trials, but focuses on the most common design type— individually randomised, two group, parallel trials. Web the consort 2010 statement is this paper including the 25 item checklist in the table ⇓ and the flow diagram ⇓. It expands the basic consort flow diagram for clinical trials to summarize external validity and. It provides guidance for reporting all randomised controlled trials, but focuses on the most common design type— individually randomised, two group, parallel trials. It provides guidance for reporting all randomised controlled trials, but focuses on the most common design type—individually randomised, two group, parallel trials. Web the consort 2010 statement is this paper including the 25 item checklist in the. Web the consort 2010 statement is this paper including the 25 item checklist in the table ⇓ and the flow diagram ⇓. The flow diagram can be accessed via the original published paper by following the pubmed links in the full bibliographic reference section of this web page. Web consort 2010 flow diagram assessed for eligibility (n= ) excluded (n=. Mary and tim created date: Web the consort 2010 statement is this paper including the 25 item checklist in the table ⇓ and the flow diagram ⇓. Web consort 2010 flow diagram assessed for eligibility (n= ) excluded (n= ) It is aimed at primary reports of rcts with two group, parallel designs. Web the expanded consort figure draws on theory, a prior meeting and recent recommendations for reporting factors related to external validity. Mary and tim last modified by: It expands the basic consort flow diagram for clinical trials to summarize external validity and contextual factors more concisely and transparently. = give reasons n =. It provides guidance for reporting all randomised controlled trials, but focuses on the most common design type—individually randomised, two group, parallel trials. Web the consort statement (or simply consort) comprises a checklist of essential items that should be included in reports of rcts and a diagram for documenting the flow of participants through a trial. Revised template of the consort diagram showing the flow of participants through each stage of a randomized trial. The flow diagram can be accessed via the original published paper by following the pubmed links in the full bibliographic reference section of this web page.
Consort Flow Diagram Template

Consort Diagram Template

Consort Flow Diagram Template Word

Consort Flow Chart Example Flowchart Examples

Consort Flow Diagram Template

Consort Flow Diagram Template

Consort Diagram Template

Consort Flow Diagram Template

Consort Flow Chart Template
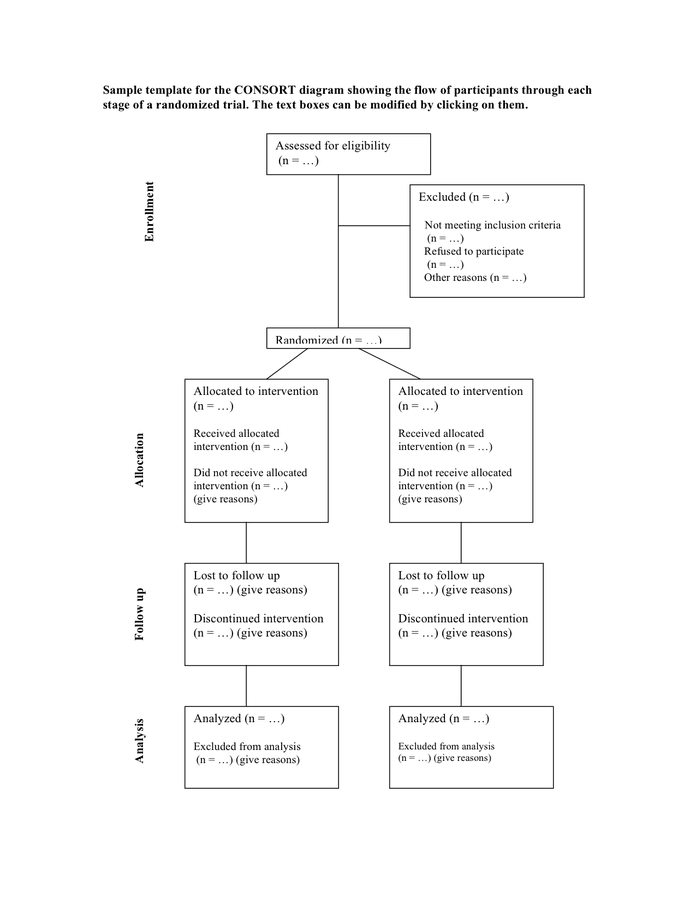
Sample template for the consort diagram in Word and Pdf formats
It Provides Guidance For Reporting All Randomised Controlled Trials, But Focuses On The Most Common Design Type— Individually Randomised, Two Group, Parallel Trials.
Analysed N = Excluded From Analysis N = Give Reasons N =.
Web The Consort 2010 Statement Is This Paper Including The 25 Item Checklist In The Table (Table 1) And The Flow Diagram (Figure 1).
Allocated To Intervention N = Received Intervention N = Did Not Receive Intervention N = Give Reasons N =.
Related Post: