Chemistry Conversion Factor Chart
Chemistry Conversion Factor Chart - Power in this case is equal to the number of times you moved. For example, the lengths of 2.54 cm and 1 in. Web c si units and conversions. Web therefore, the exact relationship of btu to joules and other energy units depends on the temperature at which btu is measured. 1 lb = 453.6 g. Web factors for unit conversions. Web select the current unit in the left column, the desired unit in the right column, and enter a value in the left column to generate the resulting conversion. 1 kg = 1000 g = 0.001 metric ton = 2.20462 lbm = 35.27392 oz. Are equivalent (by definition), and so a unit conversion factor may be derived from the ratio, Web english to metric conversions. A full list of unit conversions is available at unitconverters.net. Here are a few useful conversion factors. The conversion factors are 1 mol c2h6o over 46.07 g c2h6o, 1 mol o over 1 mol c2h6o, and 16.00 g o over 1 mole o. See the conversion factor chart. Web english to metric conversions. Web a fraction that has equivalent quantities in the numerator and the denominator but expressed in different units is called a conversion factor. Oz is a mass unit and fl oz is a volume unit in the english system. For example, if you know that there are 2.54 centimeters in every inch (or 2.2 pounds in every kilogram or 101.3. 1 lbm = 16 oz = 5 x 10‐4 ton. A full list of unit conversions is available at unitconverters.net. 1 kg = 1000 g = 0.001 metric ton = 2.20462 lbm = 35.27392 oz. Web start typing, then use the up and down arrows to select an option from the list.? Therefore, the exact relationship of btu to joules. Web an extensive set of conversion factors between the two systems of units is listed in section 5. 59 °f (15 °c) is the most widely used reference temperature for btu definition in the united states. (mass, length, volume, and area conversions are good to 4 significant figures) mass. Bold values indicate exact conversion values. The conversion factors are 1. Units in the first column are generally not used in nist publications except those that are italicized. Cubic meter ( m3 m 3) the cubic meter is defined as the volume of a cube with edges one meter in length. The conversion factors are derived from equality between the given unit and the desired unit. Web free online unit converter. 59 °f (15 °c) is the most widely used reference temperature for btu definition in the united states. Here are a few useful conversion factors. Web prepare a concept map and use the proper conversion factor. Web start typing, then use the up and down arrows to select an option from the list.? Web factors for unit conversions. At this temperature, the conversion factor is the one provided in this table. A simple conversion factor can convert meters into centimeters, or a more complex one can convert miles per hour into meters per second. To calculate the number of nails you need to build. Web conversion factors and dimensional analysis. Bold values indicate exact conversion values. Technique for doing unit conversions described in this chapter, can. Web the conversion relations in this table are commonly used to equate masses and weight assuming a nominal value for g at the surface of the earth. 8 fluid oz = 1 cup. Web converting units using a conversion diagram. Meter (m) the meter defined as the length of the. Bold values indicate exact conversion values. Morrison department of chemical engineering. Web free online unit converter to easily convert between different units of measurement for engineers, scientists and technicians. Web the only difference is the conversion factor used. 1 lb = 453.6 g. 1 in = 2.540 cm. Web therefore, the exact relationship of btu to joules and other energy units depends on the temperature at which btu is measured. See the conversion factor chart. Web a conversion factor is a factor used to convert one unit of measurement into another. At this temperature, the conversion factor is the one provided in this. A liter is exactly 1/1000 th of a cubic meter. Below is a table of conversion factors. Web factors for unit conversions. Web select the current unit in the left column, the desired unit in the right column, and enter a value in the left column to generate the resulting conversion. 1 kg = 1000 g = 0.001 metric ton = 2.20462 lbm = 35.27392 oz. (mass, length, volume, and area conversions are good to 4 significant figures) mass. 1 lbm = 16 oz = 5 x 10‐4 ton. Meter (m) the meter defined as the length of the path traveled by light in vacuum during a time interval of 1/299,792,458 of a second. si unit: Learn how to do conversions between two units in chemistry using conversion factors. Web the only difference is the conversion factor used. It would be a good idea to memorize a few conversion factors involving converting mass, volume, length and temperature. For example, the lengths of 2.54 cm and 1 in. Units in the first column are generally not used in nist publications except those that are italicized. The conversion factors are derived from equality between the given unit and the desired unit. Web a fraction that has equivalent quantities in the numerator and the denominator but expressed in different units is called a conversion factor. 1 lb = 453.6 g.
Easy Way To Remember Conversions
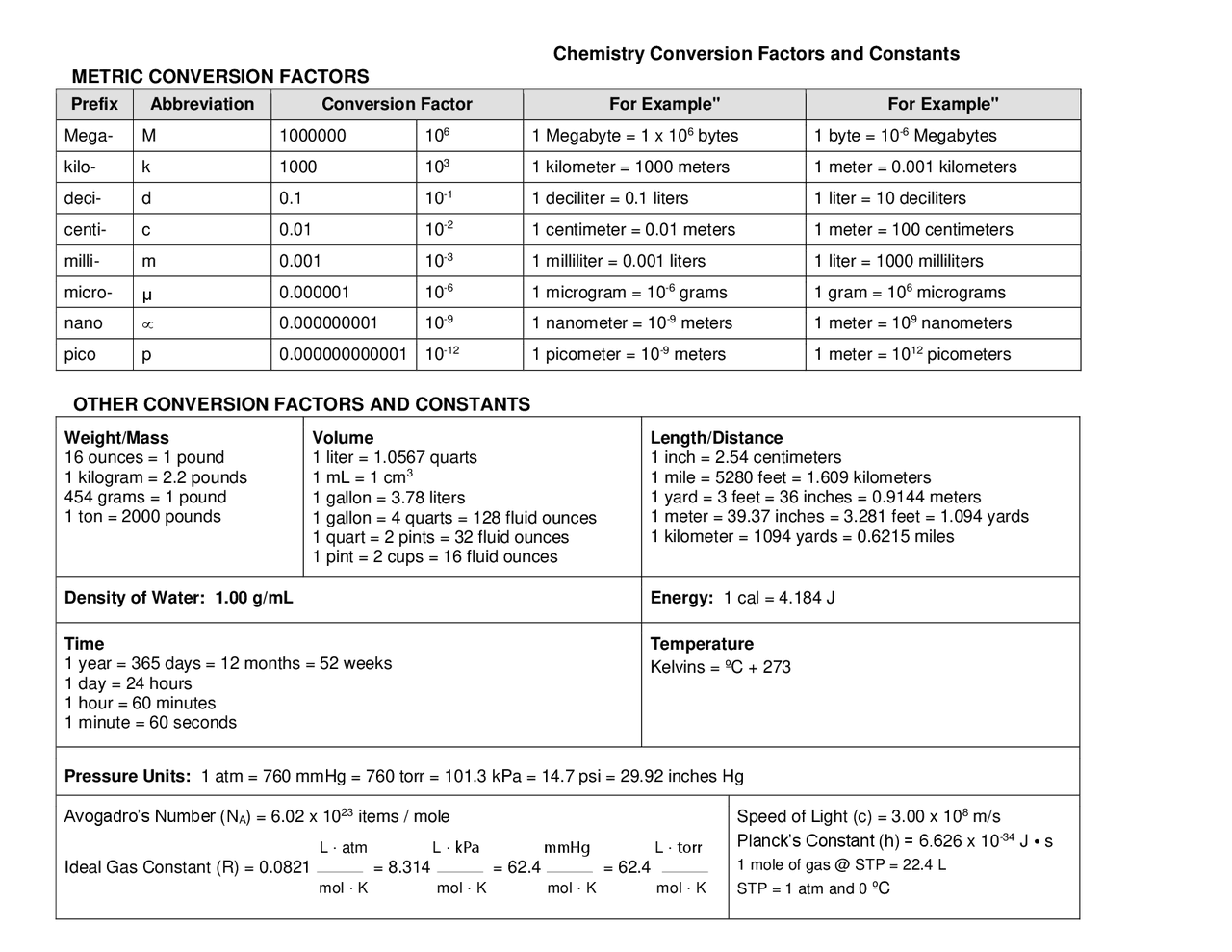
Chemistry Conversion Factors and Constants Cheat Sheet Cheat Sheet
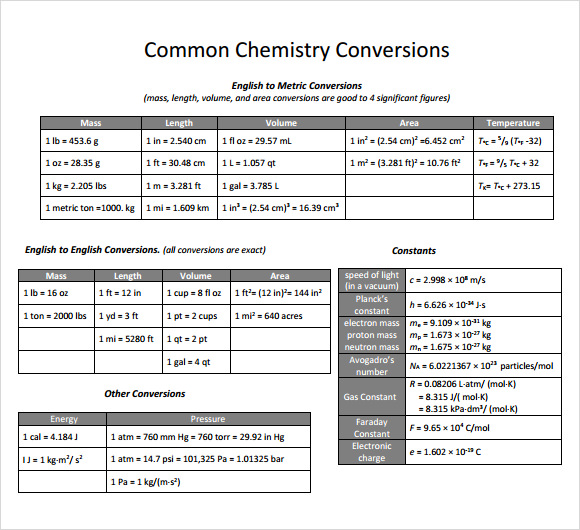
FREE 30+ Sample Metric Conversion Chart Templates in PDF, Excel, Word
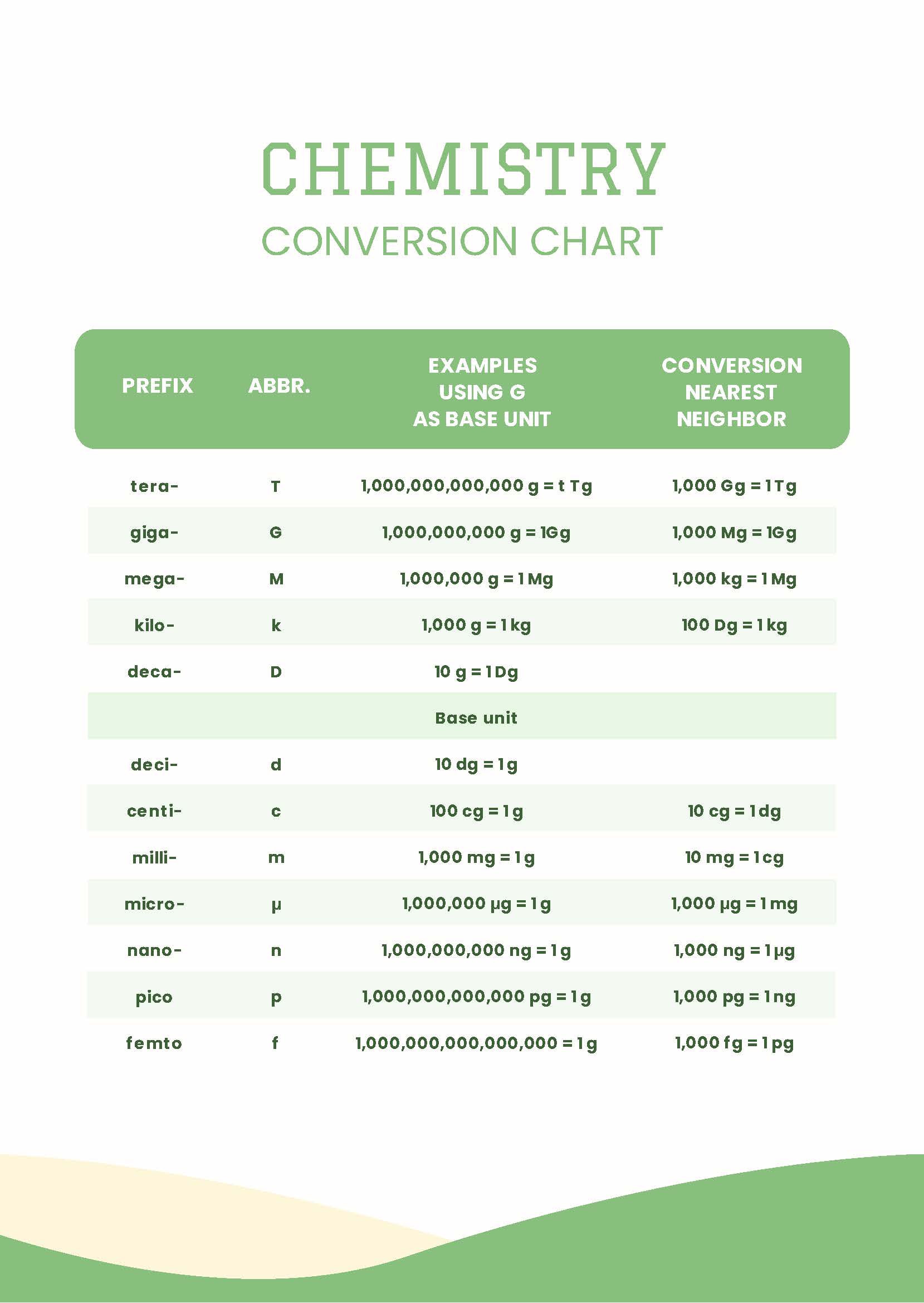
Printable Chemistry Conversion Chart

Conversion Factor Chart Chemistry
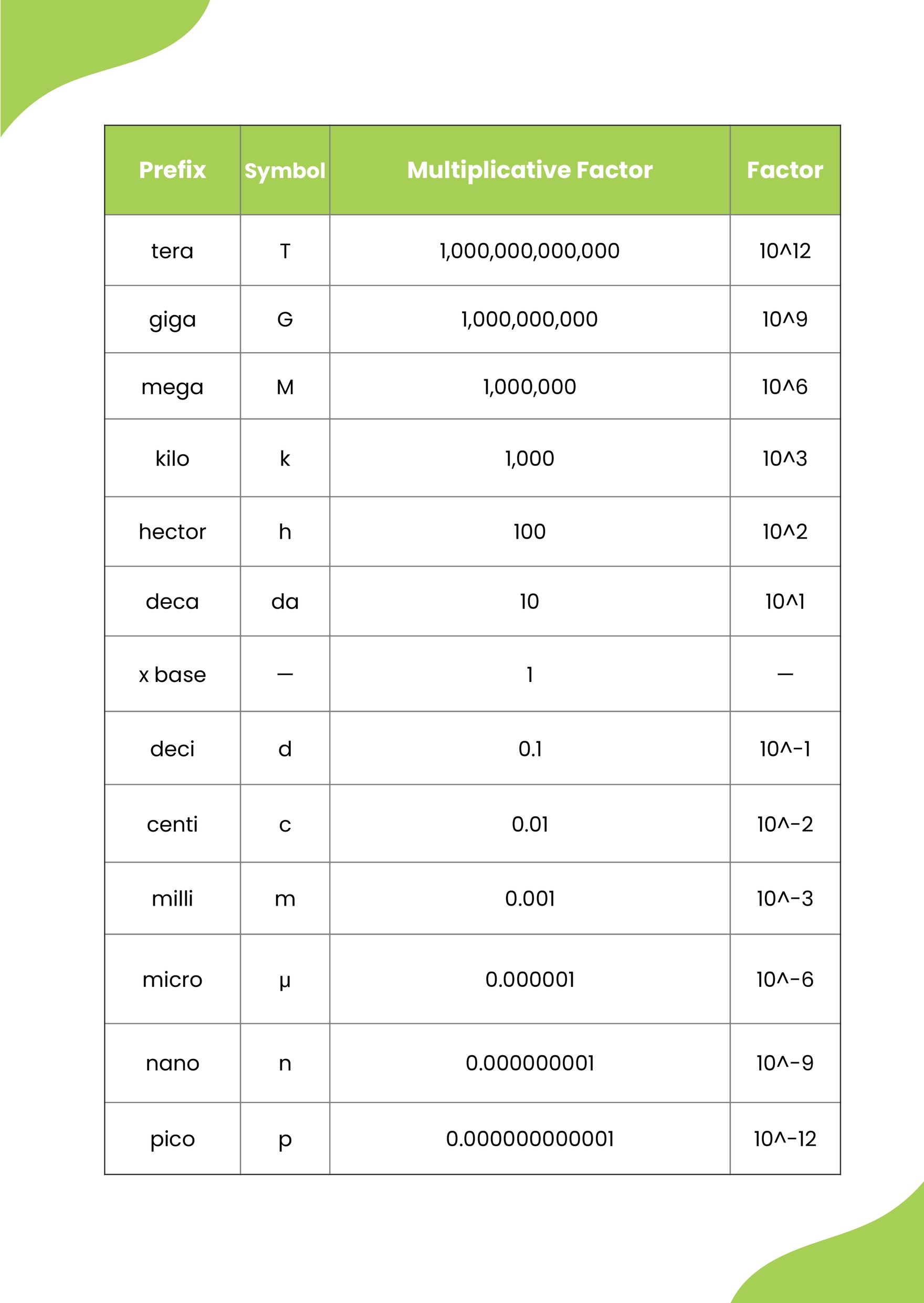
Printable Chemistry Conversion Chart
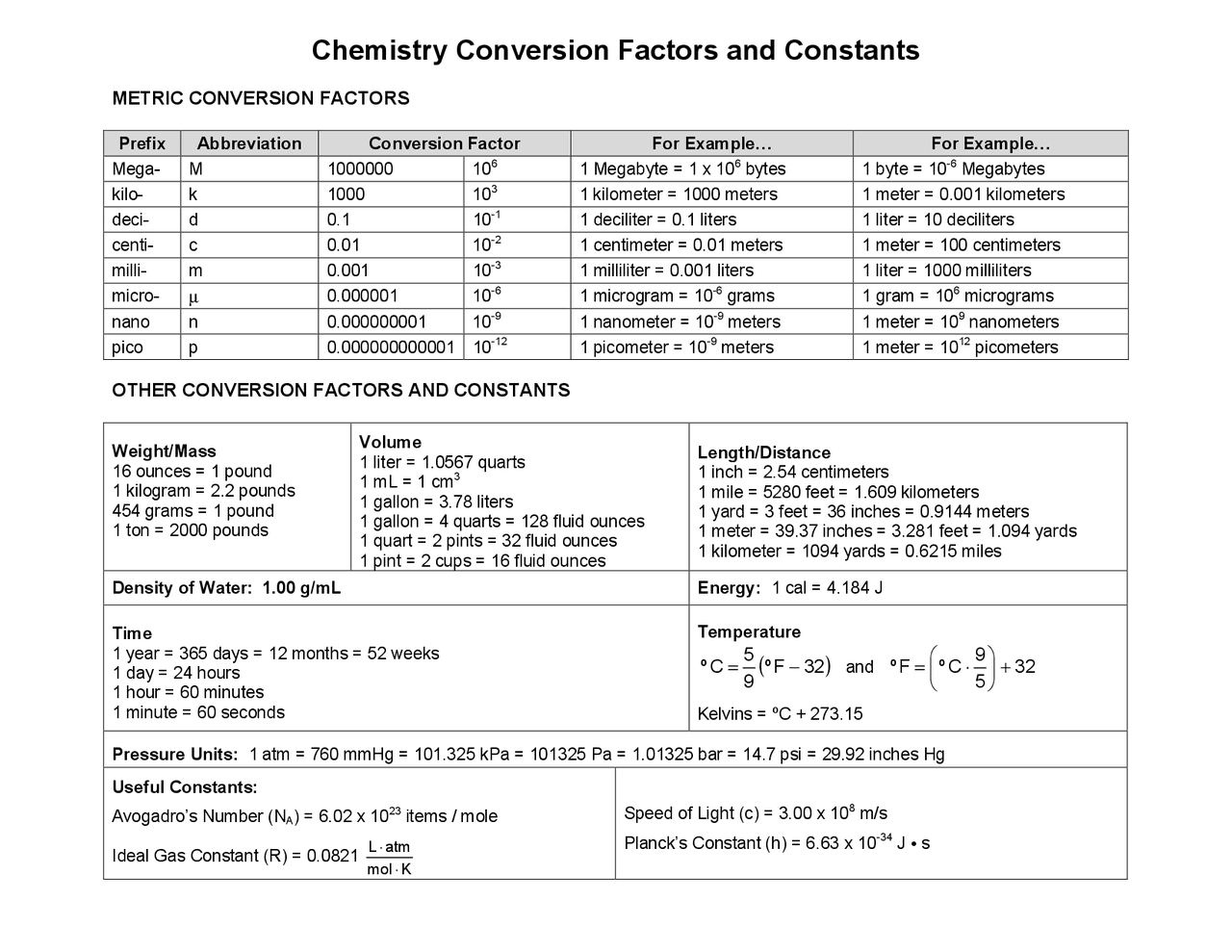
Cheat Sheet Chemistry Conversion Factors and Constants Cheat Sheet
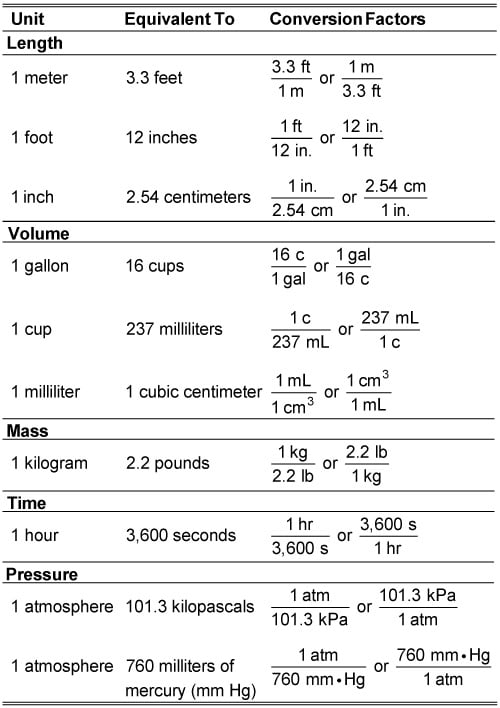
How to Convert Units Using Conversion Factors dummies
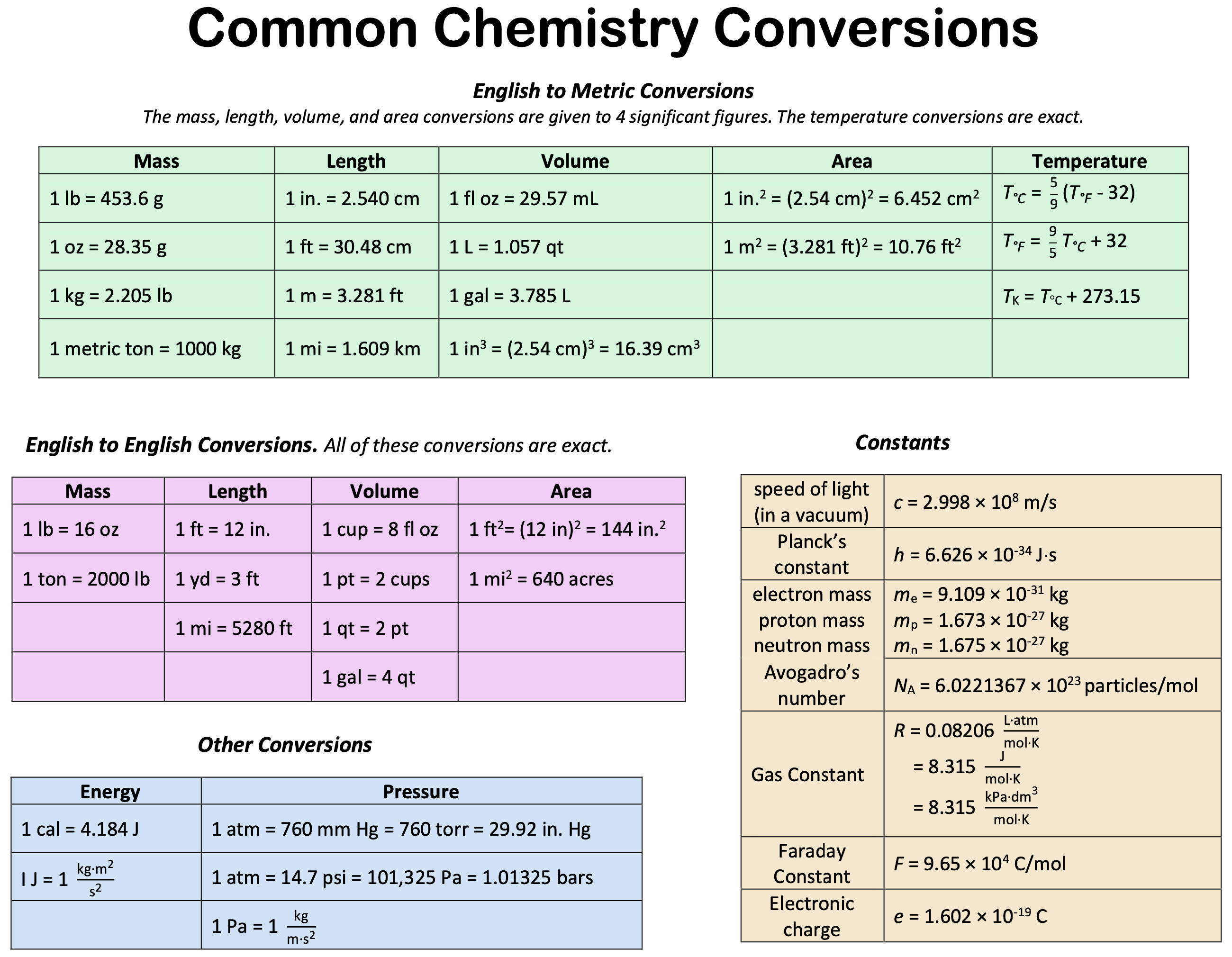
Metric Conversion Chart For Chemistry

Chemistry Conversion Factors Chart
Web What Is A Conversion Factor In Chemistry?
Power In This Case Is Equal To The Number Of Times You Moved.
Web A Conversion Factor Is A Factor Used To Convert One Unit Of Measurement Into Another.
Morrison Department Of Chemical Engineering.
Related Post: