Chart For Naming Compounds
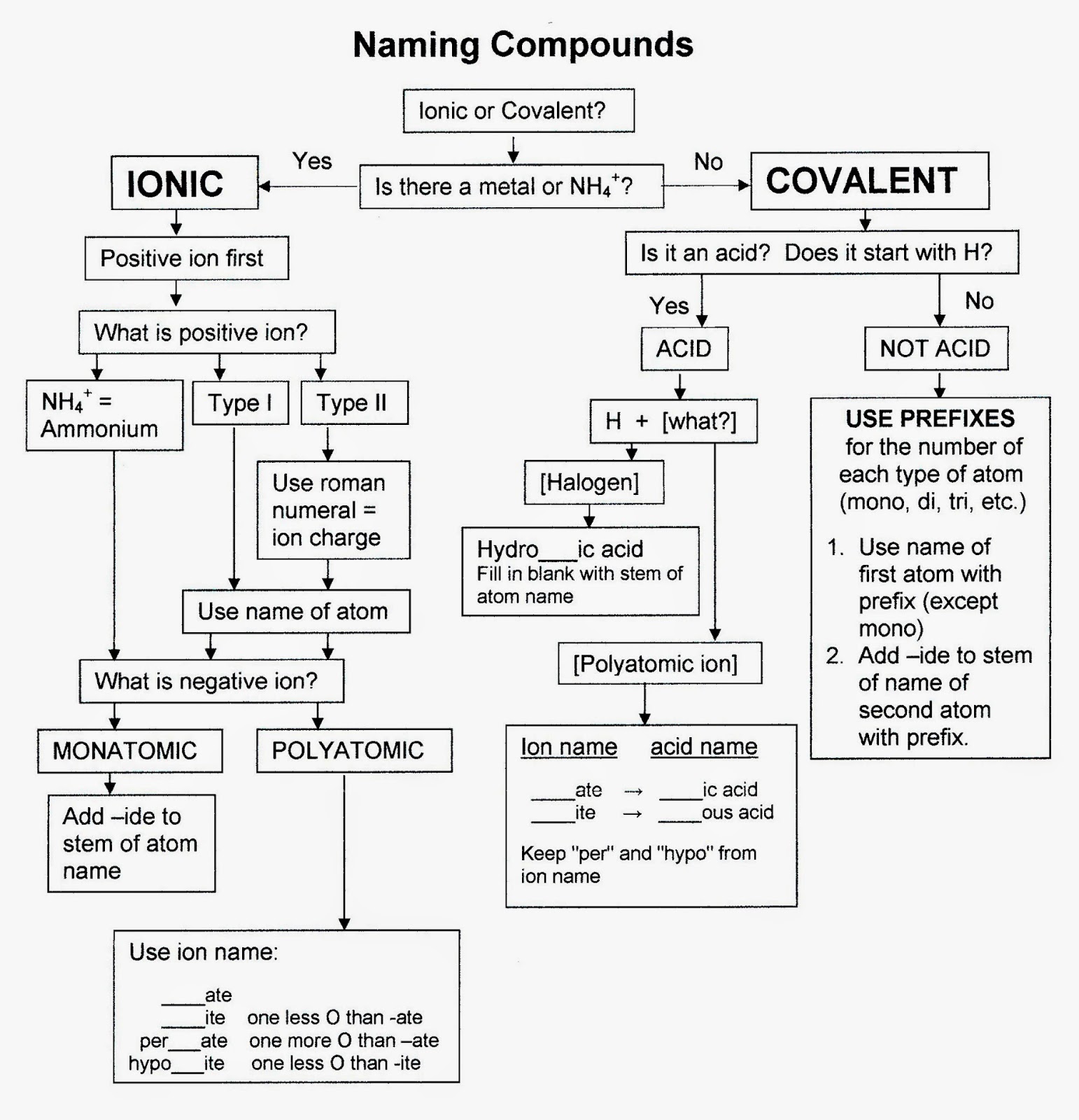
Chart For Naming Compounds - These are usually either ions or molecules; If either the cation or the anion was a polyatomic ion, the polyatomic ion name is. Web in this tutorial, you will learn all about the iupac naming of organic compounds. This includes an introduction to the iupac system of nomenclature, the steps to follow when naming an organic compound, and some practice examples. Web derive names for common types of inorganic compounds using a systematic approach. Binary (contains two elements) ionic compounds. Binary compound (metal/nonmetal) with variable charge cation: Web to name a compound, the cation name and the anion named are added together. Web naming chemical compounds from the molecular or ionic formula can be intimidating, but if you grab a periodic table and follow a few simple rules for naming compounds, you'll be a master at naming compounds in no time at all. Na + is the sodium ion and ca 2+ is the calcium ion. Today we often use chemical formulas, such as nacl, c 12 h 22 o 11, and co (nh 3) 6 (clo 4) 3 , to describe chemical compounds. Metals that form cations of more than one possible charge are named as: Binary (contains two elements) ionic compounds. Web long before chemists knew the formulas for chemical compounds, they developed a. The primary function of chemical nomenclature is to ensure that a spoken or written chemical name leaves no ambiguity concerning which chemical compound the name refers to—each chemical name should. This chapter describes an approach that is used to name simple ionic and molecular compounds, such as nacl nacl, caco3 caco 3, and n2o4 n 2 o 4. Today we. Web each compound has a name. Can combine to form compounds. Sodium chloride is an ionic compound made up of sodium ions and chloride ions in a crystal lattice. Na + is the sodium ion and ca 2+ is the calcium ion. Web naming chemical compounds from the molecular or ionic formula can be intimidating, but if you grab a. For example, hydrogen would be abbreviated as h. The primary function of chemical nomenclature is to ensure that a spoken or written chemical name leaves no ambiguity concerning which chemical compound the name refers to—each chemical name should. Acts as a single, charged unit. Start by examining the chart below and visualizing how we convert compound formulas into compound names.. Learn how to name monatomic ions and ionic compounds containing monatomic ions, predict charges for monatomic ions, and understand formulas. Web the element name + ion. Web derive names for common types of inorganic compounds using a systematic approach. Web nomenclature is the process of naming chemical compounds so that they can be easily identified as separate chemicals. When naming. Ideally, this name should indicate the composition of the compound and perhaps something of its properties. This chapter describes an approach that is used to name simple ionic and molecular compounds, such as nacl nacl, caco3 caco 3, and n2o4 n 2 o 4. If either the cation or the anion was a polyatomic ion, the polyatomic ion name is.. Binary compound (metal/nonmetal) with fixed charge cation. To name compounds, use this naming compounds flowchart (credit: For example, hydrogen would be abbreviated as h. Ideally, this name should indicate the composition of the compound and perhaps something of its properties. In this section, we discuss. Binary compound (metal/nonmetal) with variable charge cation: Acts as a single, charged unit. If either the cation or the anion was a polyatomic ion, the polyatomic ion name is. Numerical prefixes are used to specify the number of atoms in a molecule. As with ionic compounds, the system for naming covalent compounds enables chemists to write the molecular formula from. Metals that form cations of more than one possible charge are named as: Start by examining the chart below and visualizing how we convert compound formulas into compound names. Describe how to name binary covalent compounds including acids and oxyacids. Nomenclature, a collection of rules for naming things, is important in science and in many other situations. Web first, name. Nomenclature, a collection of rules for naming things, is important in science and in many other situations. These are usually either ions or molecules; Fe 2+ is the iron (ii) ion, and fe 3+ is the iron (iii) ion. The primary function of chemical nomenclature is to ensure that a spoken or written chemical name leaves no ambiguity concerning which. These are usually either ions or molecules; Acts as a single, charged unit. Web learn how to name positive ions (cations), negative ions (anions), and ionic compounds involving main group elements. Web long before chemists knew the formulas for chemical compounds, they developed a system of nomenclature that gave each compound a unique name. Such names are called systematic names and are based on a set of rules drawn up by iupac. In this section, we discuss. For example, [latex]\text{naf}[/latex] is also known as sodium fluoride. Binary compound (metal/nonmetal) with variable charge cation: Binary compound (metal/nonmetal) with variable charge cation: Web this module describes an approach that is used to name simple ionic and molecular compounds, such as nacl, caco 3, and n 2 o 4. The primary function of chemical nomenclature is to ensure that a spoken or written chemical name leaves no ambiguity concerning which chemical compound the name refers to—each chemical name should. Web first, name the nonmetal furthest to the left and bottom of the periodic table by its element name. Binary compound (metal/nonmetal) with fixed charge cation. Web each compound has a name. Different rules apply to each. As with ionic compounds, the system for naming covalent compounds enables chemists to write the molecular formula from the name and vice versa.
Naming Simple Ionic Compounds Pathways to Chemistry

Organic Chemistry Nomenclature Chart
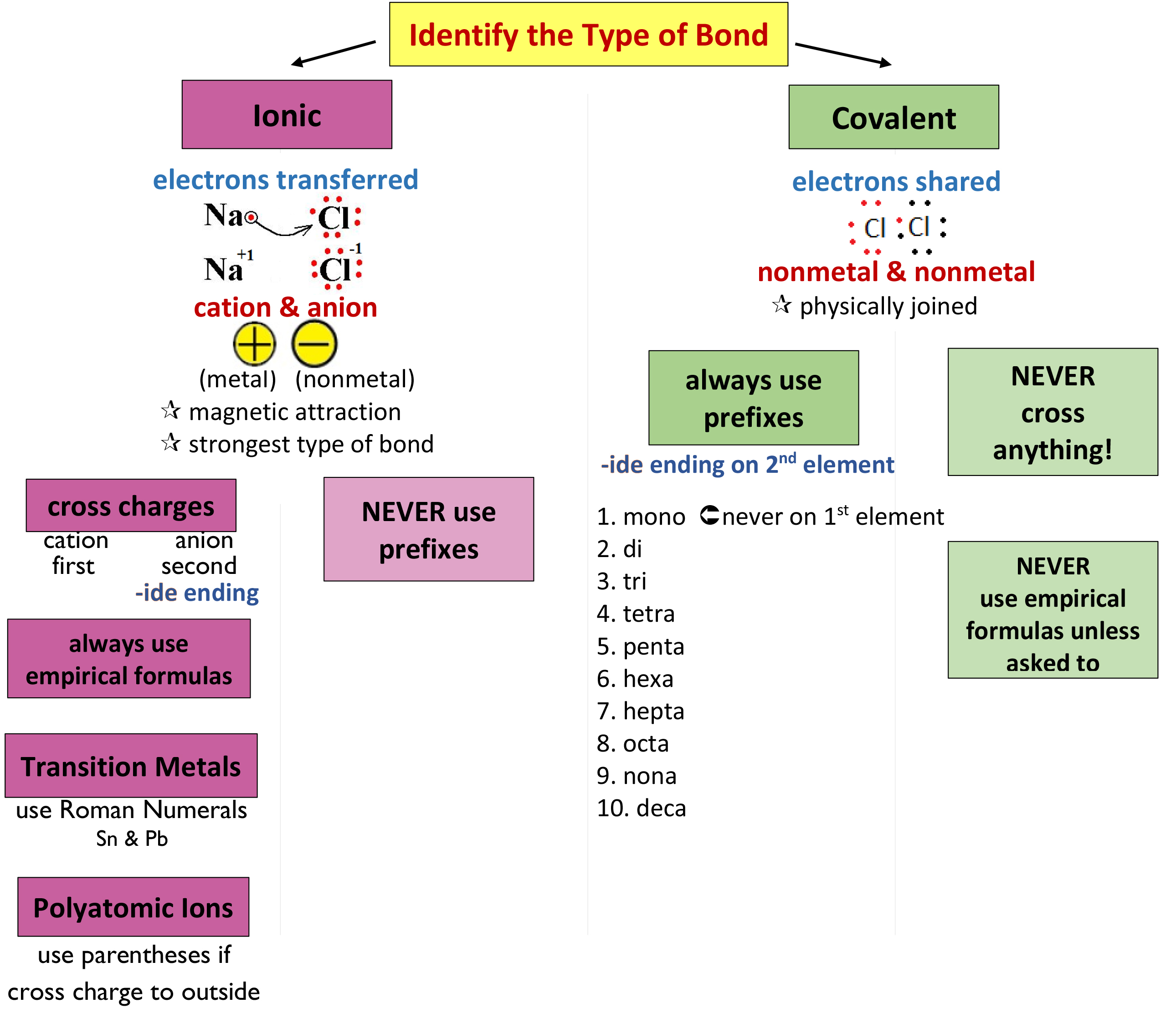
07 Nomenclature Mrs. Cook's Chemistry Class

Naming Compounds Griger Science

Chemical Names And Formulas Chart
Chemistry Naming Molecular Compounds
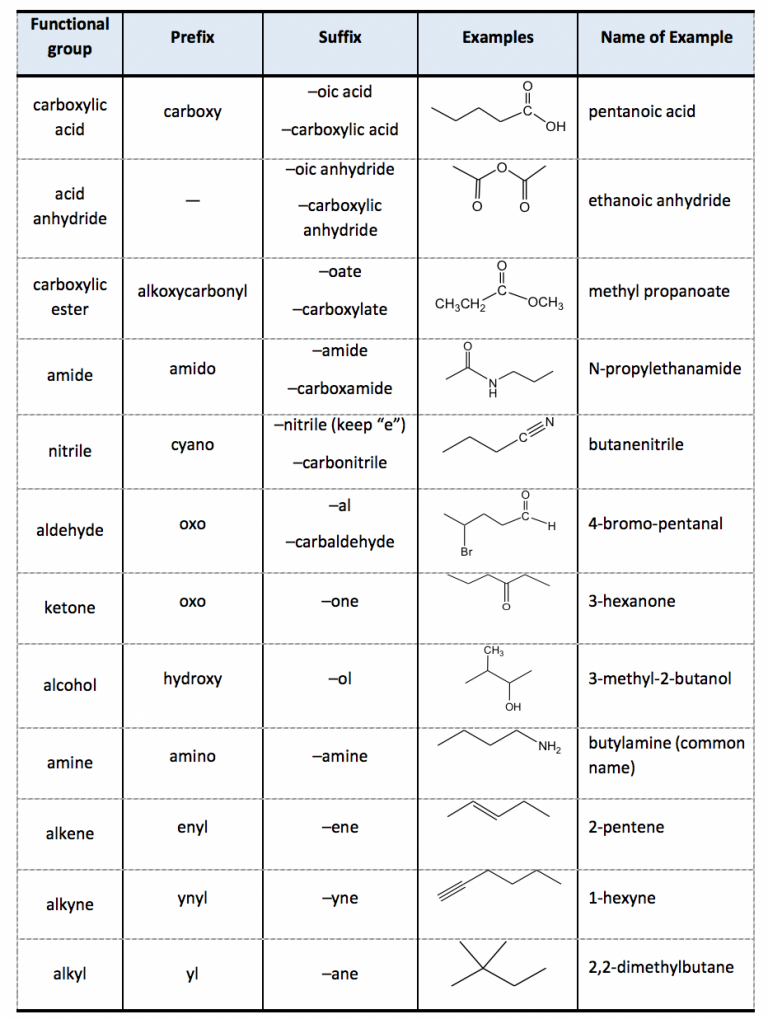
2.4 IUPAC Naming of Organic Compounds with Functional Groups Organic
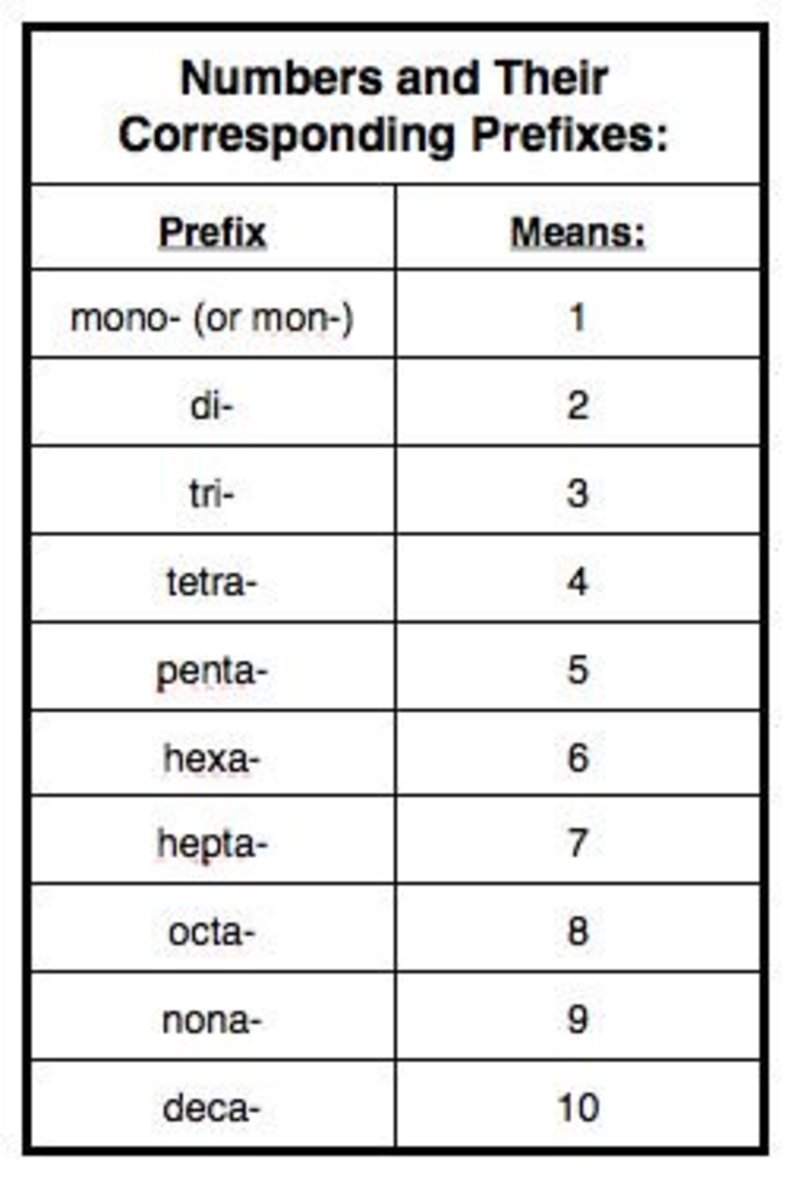
Steps to Naming Ionic and Covalent Compounds Owlcation
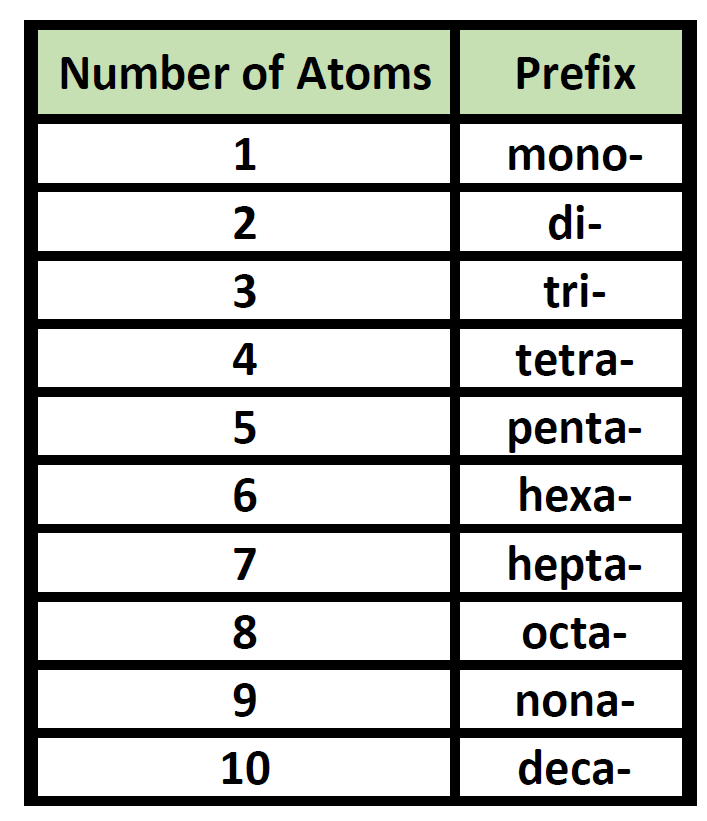
CH104 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Atoms & Compounds
Web Tutorials And Problem Sets.
Web The System Used For Naming Chemical Substances Depends On The Nature Of The Molecular Units Making Up The Compound.
Web In This Tutorial, You Will Learn All About The Iupac Naming Of Organic Compounds.
Web Can Stand Alone As Polyatomic Cation And Anion.
Related Post:
.PNG)