Cations And Anions Chart
Cations And Anions Chart - That is, group 1 elements form 1+. Web here is a table of common anions and a description of how to write formulas for cation and anion compounds. A cation has fewer electrons than protons. Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or. Web cations are positively charged ions formed when neutral atoms lose electrons; Cations have a positive charge. Web if more protons are present, the ion is positive and is known as a cation; If more electrons are present, the ion is negative and referred to as an anion. Web in this tutorial, you will learn about the properties, differences, and examples of ions, cations and anions, as well as how to predict them based on their positions on the. When a neutral atom loses one or more electrons, the total number of electrons decreases while the number of protons in the nucleus remains the same. For example, iron(ii) has a 2+ charge; Typical cations include sodium (na+), calcium (ca++ = ca 2+ ), and. Web cations are positively charged ions formed when neutral atoms lose electrons; If more electrons are present, the ion is negative and referred to as an anion. See a visual chart of. Cations and anions may consist of single atoms or. For example, iron(ii) has a 2+ charge; Web in this tutorial, you will learn about the properties, differences, and examples of ions, cations and anions, as well as how to predict them based on their positions on the. Roman numeral notation indicates charge of ion when element commonly forms more than. An ion consisting of two. Web in this tutorial, you will learn about the properties, differences, and examples of ions, cations and anions, as well as how to predict them based on their positions on the. That is, group 1 elements form 1+. Cations have a positive charge. Ions are divided into two groups; Atoms do this so that they can. Then, identify the anion and write down its symbol and charge. A cation has fewer electrons than protons. Web a table of common cations and anions with their formulas, charges and names. Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and. Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or. Web cations are positively charged ions formed when neutral atoms lose electrons; Web in this tutorial, you will learn about the properties, differences, and examples of. Web if an atom loses electrons, it becomes a positive ion known as a cation, which has fewer electrons than protons and thus has a positive charge. Web here is a table of common anions and a description of how to write formulas for cation and anion compounds. An ion consisting of a single atom is a monatomic ion; Web. Ions are divided into two groups; Web cations are positively charged ions formed when neutral atoms lose electrons; Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or. Then, identify the anion and write down its. Web cations are positively charged ions formed when neutral atoms lose electrons; Then, identify the anion and write down its symbol and charge. Web if an atom loses electrons, it becomes a positive ion known as a cation, which has fewer electrons than protons and thus has a positive charge. That is, group 1 elements form 1+. Web cations are. Ions are divided into two groups; For example, iron(ii) has a 2+ charge; Typical cations include sodium (na+), calcium (ca++ = ca 2+ ), and. Web cations are ions which have a positive electrical charge. An ion consisting of a single atom is a monatomic ion; Typical cations include sodium (na+), calcium (ca++ = ca 2+ ), and. Web in this tutorial, you will learn about the properties, differences, and examples of ions, cations and anions, as well as how to predict them based on their positions on the. Web cations are ions which have a positive electrical charge. Web common covalent binary inorganic compounds #. Includes notes about hydrogen, hydronium and mercury ions. Web if an atom loses electrons, it becomes a positive ion known as a cation, which has fewer electrons than protons and thus has a positive charge. Cations have a positive charge. Web if more protons are present, the ion is positive and is known as a cation; An ion consisting of a single atom is a monatomic ion; An ion consisting of two. Atoms do this so that they can. Web in this tutorial, you will learn about the properties, differences, and examples of ions, cations and anions, as well as how to predict them based on their positions on the. That is, group 1 elements form 1+. Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or. Anions are negatively charged ions formed when neutral atoms gain electrons. Web cations are positively charged ions formed when neutral atoms lose electrons; Web a table of common cations and anions with their formulas, charges and names. Cations and anions may consist of single atoms or. An ion may consist of a single atom of an element ( a monatomic. For example, iron(ii) has a 2+ charge;
naming anions and cations worksheet

Periodic Table List Of Cations And Anions Periodic Table Timeline
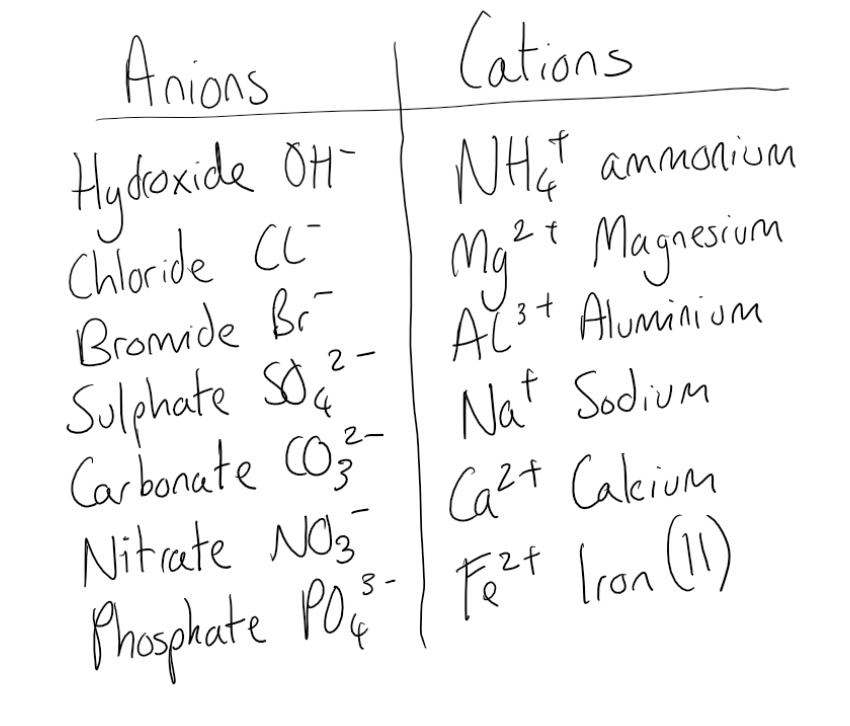
Anion Cation Common Names Formulas And Charges For Familiar Ions Ions

Cations and Anions Definitions, Examples, and Differences

Table Of Cations And Anions
Common Ions Anions and Cations PDF

Anion And Cation Chart

Ion Names, Formulas and Charges Chart Teaching chemistry, Chemistry
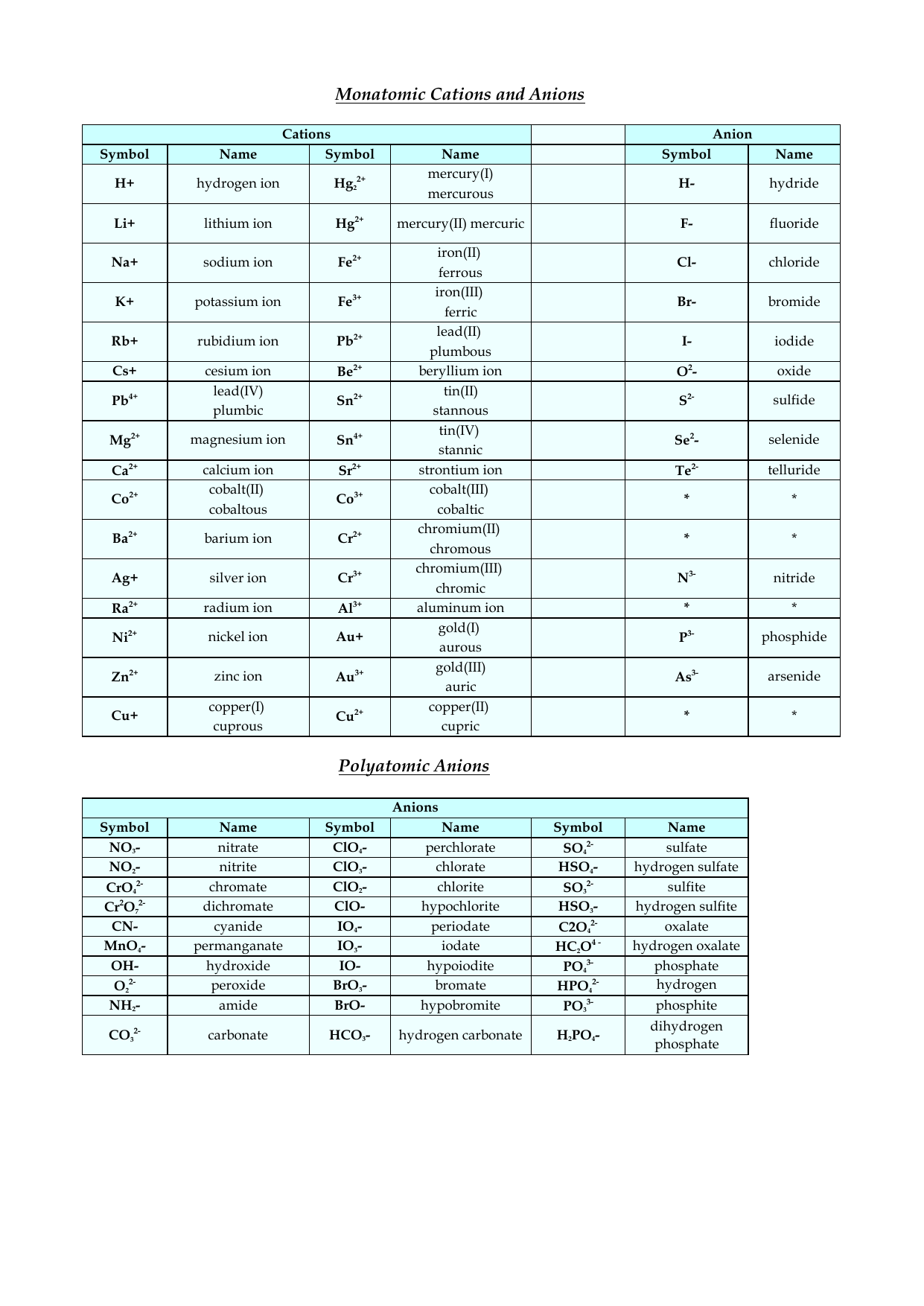
Tabela De Cations E Anion
List of Cations and Anions PDF Ion Hydrogen
Web To Find The Formula Of An Ionic Compound, First Identify The Cation And Write Down Its Symbol And Charge.
Web Common Covalent Binary Inorganic Compounds # Of Atoms Prefix (Element Closest To Fluorine Goes On Right)Common Examples 1 Mono H 2 Hydrogen N 2 Nitrogen 2 Di O 2.
If More Electrons Are Present, The Ion Is Negative And Referred To As An Anion.
Web Here Is A Table Of Common Anions And A Description Of How To Write Formulas For Cation And Anion Compounds.
Related Post:

